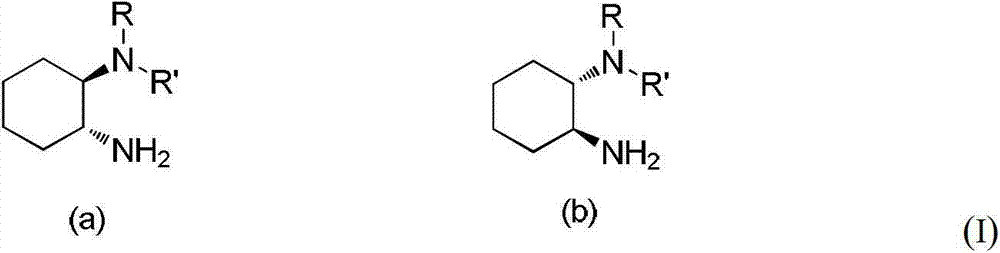
Chirality N, N-dialkyl-1, 2-diaminocyclohexane catalyst as well as preparation method and application thereof
A cyclohexanediamine catalyst and cyclohexanediamine technology are used in the preparation of amino compounds, the preparation of carbon-based compounds, chemical instruments and methods, etc., to achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
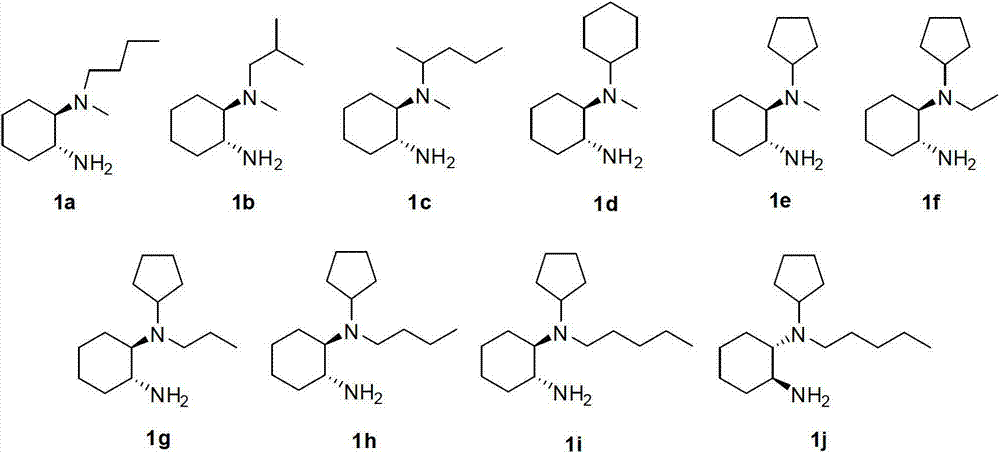
[0035] Example 1. (1R,2R)-N 1 -Butyl-N 1 - Preparation of methyl-1,2-cyclohexanediamine dihydrochloride (1a·2HCl).
[0036] (1R,2R)-N 1 -Butyl-N 1 -The structural formula of methyl-1,2-cyclohexanediamine dihydrochloride (1a 2HCl) is as follows:
[0037]
[0038] Concrete preparation steps are as follows:
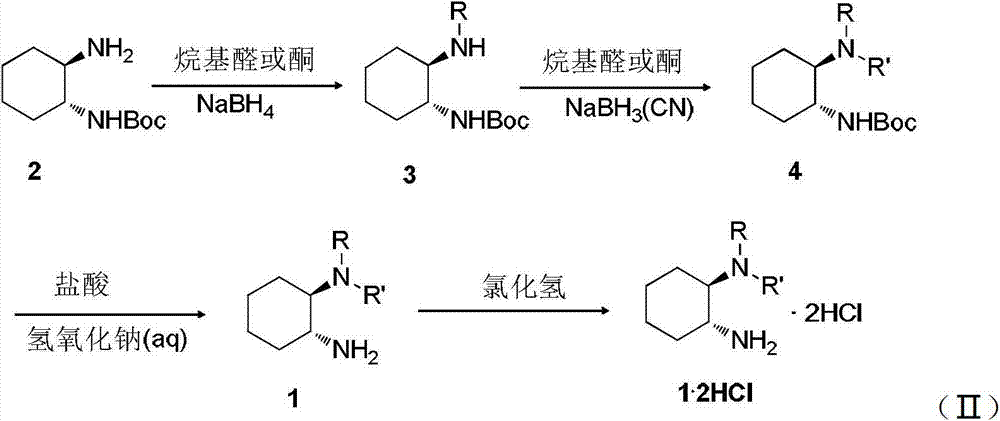
[0039] Add 10mmol of (1R,2R)-1,2-cyclohexanediamine protected by mono-tert-butoxycarbonyl and 50mmol of n-butyraldehyde into 50mL of methanol, and react at 0~20°C for 4~5 hours. The reaction solution was cooled in an ice bath, and then 30 mmol NaBH 4 Add in batches, continue to react for 0.5 hours, and then heat to 25°C for 3 hours to obtain intermediate 3a (the product does not need to be isolated). Add 20mmolNaBH to the reaction solution 3 (CN), 2.4mL formaldehyde solution with mass percent concentration of 37%, reacted at 30°C for 5 hours. The solvent was removed under reduced pressure, 40 mL of 1 mol / L NaOH aqueous solution was added, and 50 mL of ethyl acetate ...
Embodiment 2
[0042] Example 2. (1R,2R)-N 1 -Isobutyl-N 1 - Preparation of methyl-1,2-cyclohexanediamine dihydrochloride (1b·2HCl).
[0043] (1R,2R)-N 1 -Isobutyl-N 1 -The structural formula of methyl-1,2-cyclohexanediamine dihydrochloride (1b 2HCl) is as follows:
[0044]
[0045] Concrete preparation steps are as follows:
[0046] Add 10mmol of (1R,2R)-1,2-cyclohexanediamine protected by mono-tert-butoxycarbonyl and 50mmol of isobutyraldehyde into 50mL of methanol solution, and react at 20~40°C for 3~4 hours. The reaction solution was cooled in an ice bath, and then 30 mmol NaBH 4 Add in batches, continue to react for 1.5 hours, and then heat to 40°C for 2 hours to obtain intermediate 3b (the product does not need to be isolated). Add 20mmolNaBH to the reaction solution 3 (CN), 2.4mL formaldehyde solution with mass percent concentration of 37%, reacted at 25°C for 8 hours. The solvent was removed under reduced pressure, 40 mL of 1 mol / L NaOH aqueous solution was added, and 50 m...
Embodiment 3
[0049] Example 3. (1R,2R)-N 1 -(2-Pentyl)-N 1 - Preparation of methyl-1,2-cyclohexanediamine dihydrochloride (1c·2HCl).
[0050] (1R,2R)-N 1 -(2-Pentyl)-N 1 -The structural formula of methyl-1,2-cyclohexanediamine dihydrochloride (1c 2HCl) is as follows:
[0051]
[0052] Concrete preparation steps are as follows:
[0053] Add 10mmol of (1R,2R)-1,2-cyclohexanediamine protected by mono-tert-butoxycarbonyl and 50mmol of 2-pentanone into 50mL of methanol solution, and react at 40~60°C for 2~3 hours. The reaction solution was cooled in an ice bath, and then 30 mmol NaBH 4 Added in batches, continued to react for 2 hours, and then heated to 60°C for 0.5 hours to obtain intermediate 3c (the product does not need to be isolated). Add 20mmolNaBH to the reaction solution 3 (CN), 2.4mL formaldehyde solution with mass percent concentration of 37%, reacted at 20°C for 12 hours. The solvent was removed under reduced pressure, 40 mL of 1 mol / L NaOH aqueous solution was added, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com