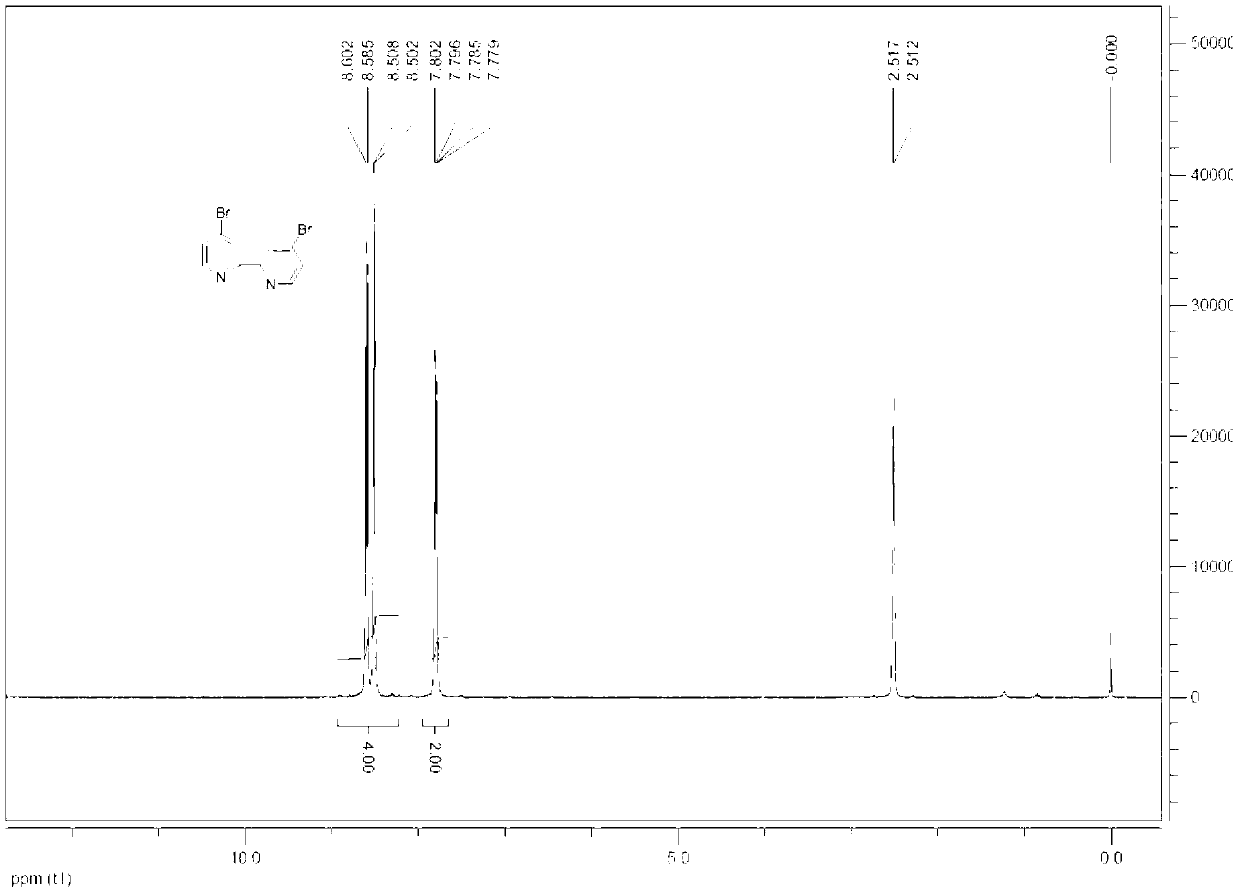
Synthesis method for 4, 4'-dibromo-2, 2'-dipyridyl
A synthetic method, bipyridine technology, applied in the direction of organic chemistry, can solve problems such as inappropriateness, achieve the effects of increased yield, simplified process, and reduced adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
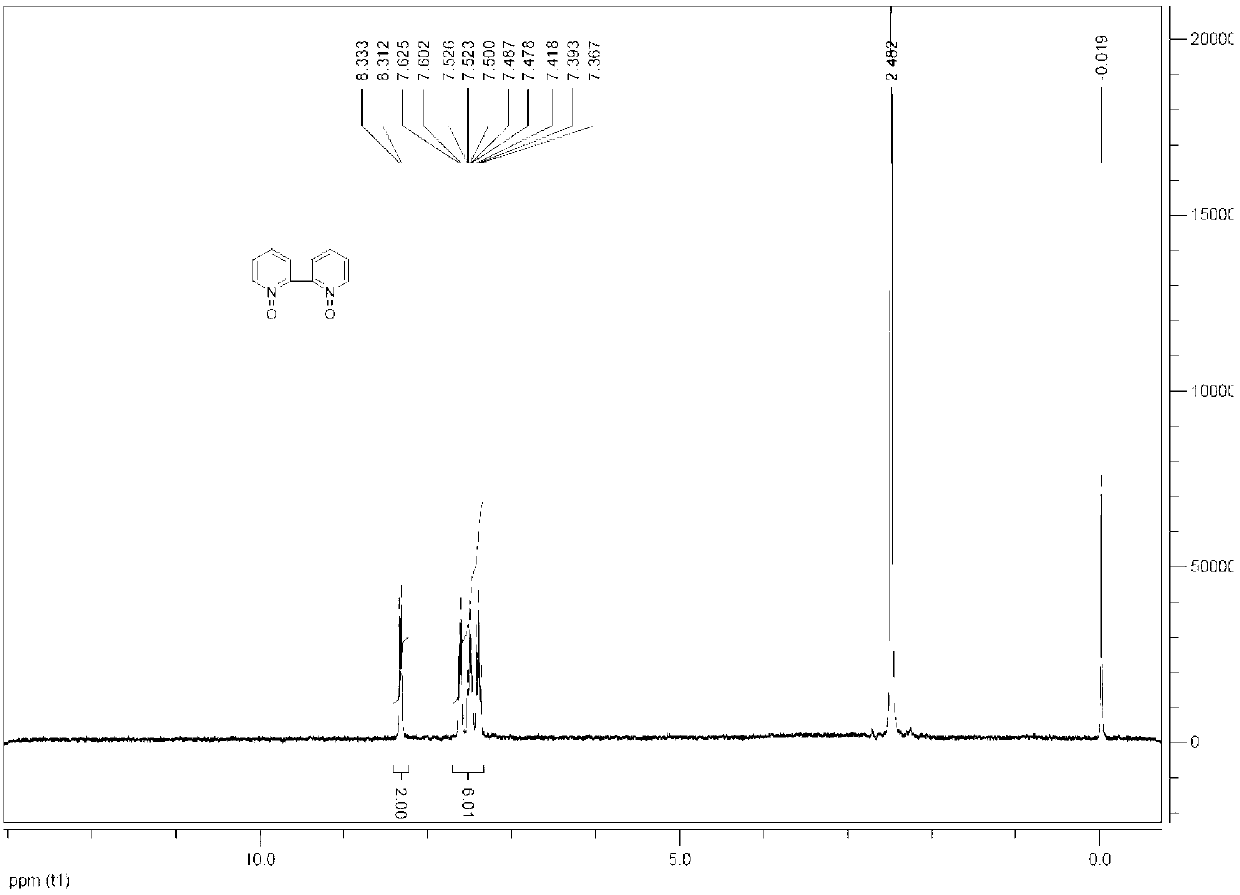
[0045]Add 50ml of water to a 100ml four-necked flask, then add 7.5g (48mmol) 2,2'-dipyridine, add 1.6g (0.0048mmol) sodium tungstate and 2.2g (0.0048mmol) trioctyl ammonium methyl hydrogen sulfate, Raise the temperature to 60°C, slowly add 18.75ml (182.4mmol) of 30% hydrogen peroxide dropwise, react for 5h, no raw materials were detected by HPLC, filter, wash with water three times, and dry to obtain 7.2g of 2,2'-bipyridine nitrogen oxide, the yield 80%, purity 99.4% (HPLC). 1H-NMR(DMSO-d6) of 2,2'-bipyridine nitrogen oxide: 8.31-8.33 (d, J=6.3Hz, 2H) 7.39-7.62 (m, 6H), such as figure 1 shown.
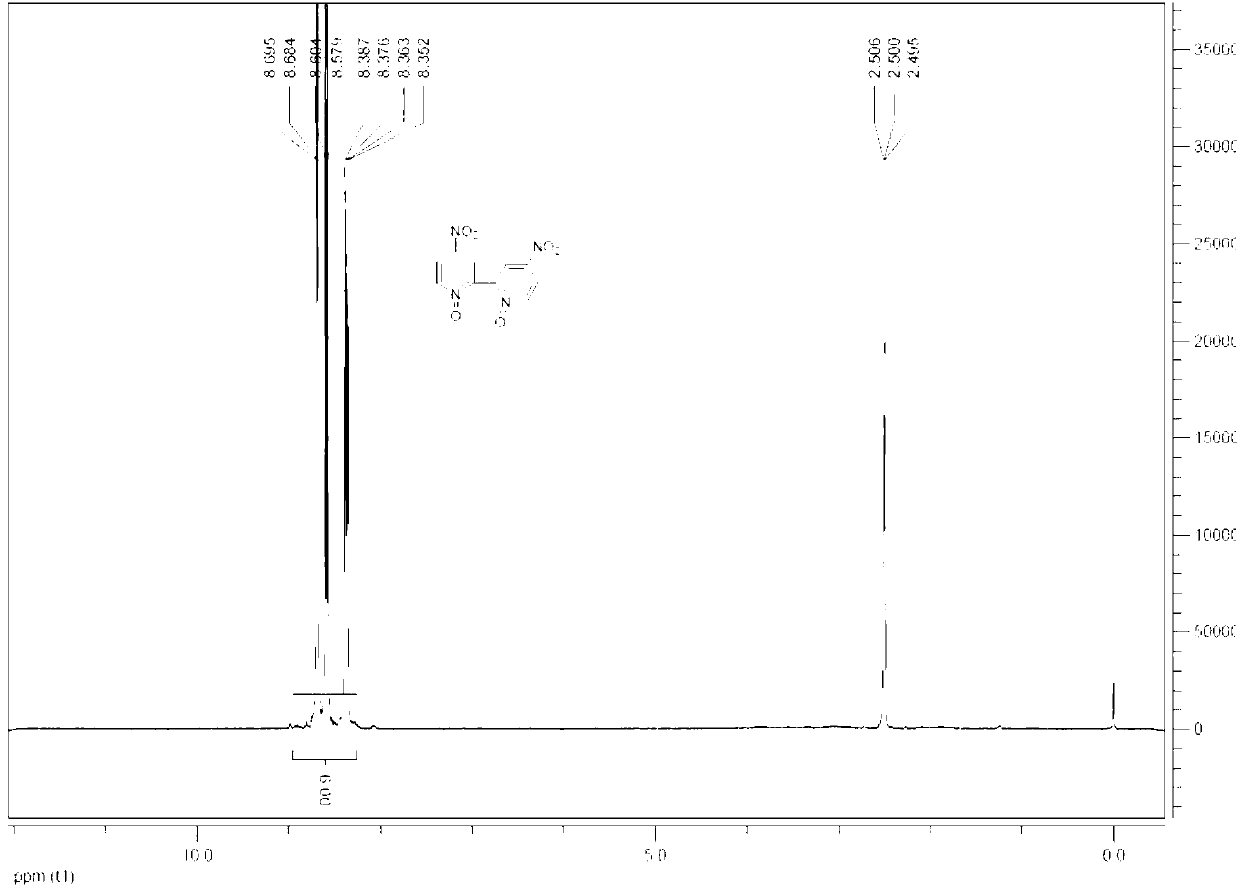
[0046] Add 35ml (657mmol) of concentrated sulfuric acid and 40ml of 30% oleum into a 250ml four-neck flask, add 25g (133mmol) of 2,2'-bipyridine nitrogen oxide in batches, raise the temperature to 110°C, add dropwise 80ml (1942mmol) of fuming Nitric acid, reacted for 5h after the dropwise addition, and monitored by HPLC. The reaction solution was poured into ice water, filtered, rec...
Embodiment 2
[0049] Add 50ml of water to a 100ml four-necked flask, then add 7.5g (48mmol) 2,2'-dipyridine, add 0.8g (0.0024mmol) sodium tungstate and 1.1g (0.0024mmol) trioctylmethyl ammonium bisulfate, The temperature was raised to 70°C. Slowly add 18.75ml (182.4mmol) 30% hydrogen peroxide dropwise, react for 8 hours, no raw materials are detected by HPLC, filter, wash with water three times, and dry to obtain 7.0g of 2,2'-bipyridine nitrogen oxide, yield 77%, purity 99.6 % (HPLC).
[0050] Add 35ml (657mmol) of concentrated sulfuric acid and 40ml of 30% oleum into a 250ml four-neck flask, add 25g (133mmol) of 2,2'-bipyridine nitrogen oxide in batches, raise the temperature to 120°C, add dropwise 80ml (1942mmol) of fuming Nitric acid, reacted for 5h after the dropwise addition, and monitored by HPLC. The reaction solution was poured into ice water, filtered, recrystallized, and dried to obtain 18.4 g of a yellow solid with a yield of 50.0% and a purity of 99.3%.
[0051] In a 250ml fo...
Embodiment 3
[0053] Add 50ml of water to a 100ml four-neck flask, add 7.5g (48mmol) of 2,2'-bipyridine, add 0.82g of sodium molybdate and 1.35g of trioctylmethylammonium chloride, and heat up to 65°C. Slowly add 18.75ml (182.4mmol) of 30% hydrogen peroxide dropwise, react for 5 hours, no raw material is detected by HPLC, filter, wash with water three times, and dry to obtain 7.1g of 2,2'-bipyridine nitrogen oxide, with a yield of 78.1% and a purity of 99.5 % (HPLC).
[0054] Add 35ml (657mmol) of concentrated sulfuric acid and 40ml of 30% oleum into a 250ml four-neck flask, add 25g (133mmol) of 2,2'-bipyridyl nitrogen oxide in batches, raise the temperature to 110~120°C, and add 80ml (1942mmol) dropwise Fuming nitric acid, react for 5 hours after the dropwise addition, and monitor by HPLC. The reaction solution was poured into ice water, filtered, recrystallized, and dried to obtain 18.4 g of a yellow solid with a yield of 50% and a purity of 99.3%.
[0055] In a 250ml four-necked flask,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com