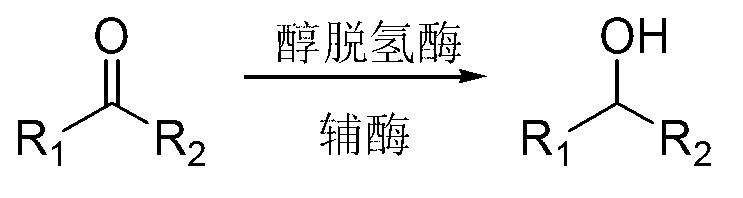
Method for reducing ketone compound by using two alcohol dehydrogenases
A technology of ketone compounds and alcohol dehydrogenase, which is applied in the field of reducing ketone compounds with two kinds of alcohol dehydrogenase, can solve the problems of limited application range, harsh reaction conditions, unnatural products, etc., and achieve safe and stable process conditions , mild reaction conditions and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
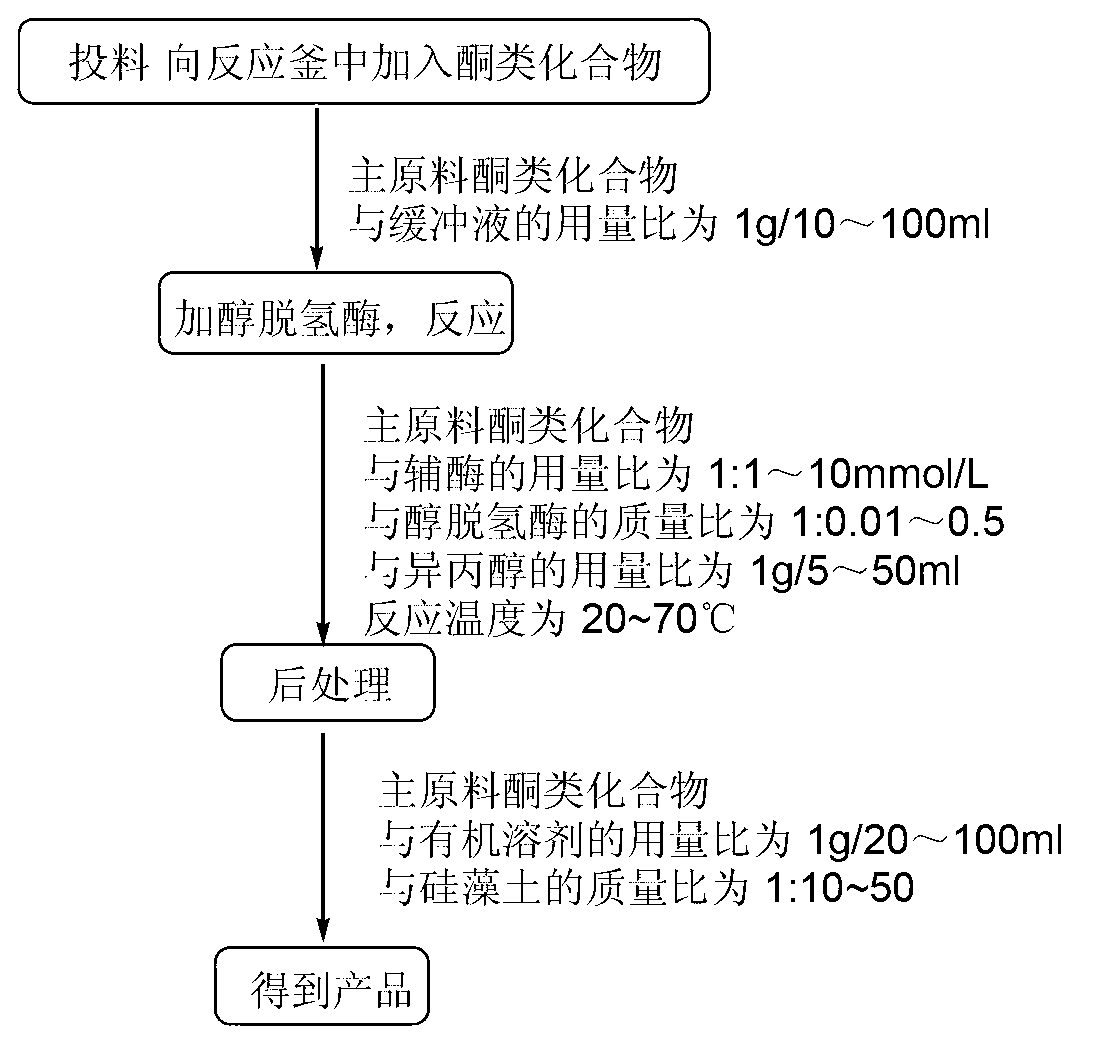
[0024] Embodiment 1: the method for applying two kinds of alcohol dehydrogenases to reduce ketones is characterized in that the specific steps are as follows:
[0025] (1) Feeding: Add 5g (1eq) of the main raw material 3,4-dimethoxypropiophenone into a 250ml Erlenmeyer flask 50ml (10ml / g) phosphate buffer (50mmol / LpH=6.0), so that the substrate is evenly dispersed in the buffer;
[0026] (2) Alcohol dehydrogenase: continue to add 0.033g (0.0066g / g) reduced nicotinamide adenine dinucleotide, 0.05g (0.01g / g) alcohol dehydrogenase PAR and 25ml (5ml / g) of isopropanol, system pH=6.0;
[0027] (3) Reaction: The system was reacted in a shaker at 150rpm, and kept at 20±3°C for 24h;
[0028] (4) Post-treatment: filter the system with 50g (10g / g) of diatomaceous earth, extract with 100ml (20ml / g) of ethyl acetate, let stand to separate the liquid, dry the organic phase, filter, concentrate to obtain the crude product, and then pass through the column layer Analyze and purify to obta...
Embodiment 2
[0030] Embodiment 2: the method for applying two kinds of alcohol dehydrogenases to reduce ketones is characterized in that the specific steps are as follows:
[0031] (1) Feeding: Add 2g (1eq) of the main raw material N-tert-butoxycarbonyl-3-piperidone into a 250ml Erlenmeyer flask 40ml (20ml / g) phosphate buffer (50mmol / L, pH=7.0), to disperse the substrate evenly in the buffer;
[0032] (2) Alcohol dehydrogenase: continue to add 0.198g (0.099g / g) reduced nicotinamide adenine dinucleotide, 0.12g (0.06g / g) alcohol dehydrogenase PAR and 16ml (8ml / g) of isopropanol, system pH=7.0;
[0033] (3) Reaction: The system was reacted in a shaker at 200rpm, and kept at 20±3°C for 32h;
[0034] (4) Post-treatment: filter the system with 40g (20g / g) diatomaceous earth, extract with 60ml (30ml / g) dichloromethane, let stand to separate the liquid, dry the organic phase, filter, concentrate to get the crude product, and then pass through the column layer Analyze and purify to obtain the h...
Embodiment 3
[0035] Embodiment 3: the method for applying two kinds of alcohol dehydrogenases to reduce ketones is characterized in that the specific steps are as follows:
[0036] (1) Feeding: Add 2g (1eq) of the main raw material p-methylacetophenone into a 500ml Erlenmeyer flask 100ml (50ml / g) tris buffer (50mmol / L, pH=8.0), the substrate is evenly dispersed in the buffer;
[0037] (2) Alcohol dehydrogenase: Continue to add 0.248g (0.124g / g) reduced nicotinamide adenine dinucleotide phosphate, 0.2g (0.1g / g) alcohol dehydrogenase TeSADH and 40ml (20ml / g) of isopropanol, system pH=8.0;
[0038] (3) Reaction: The system was reacted in a shaker at 200rpm, and kept at 30±5°C for 45h;
[0039] (4) Post-treatment: filter the system with 50g (25g / g) diatomaceous earth, extract with 120ml (60ml / g) ethyl acetate, let stand to separate the liquid, dry the organic phase, filter, concentrate to get the crude product, and then pass through the column layer Analyze and purify to obtain 1.92g produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com