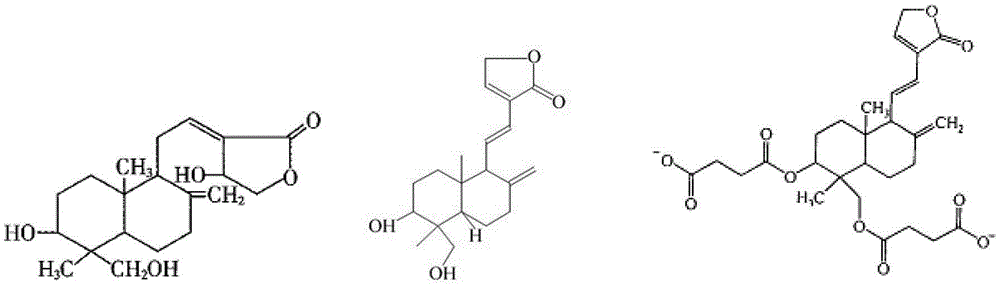
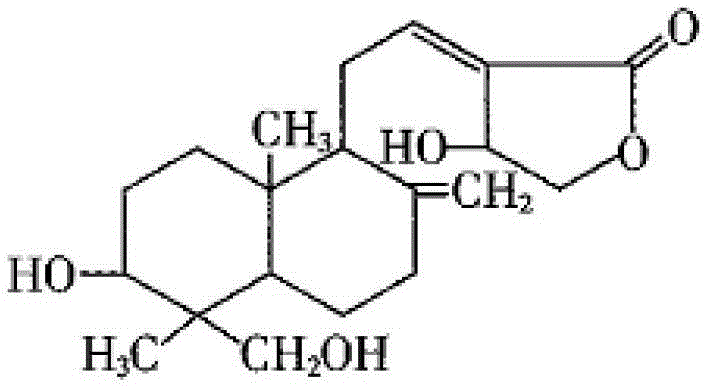
New crystal form of andrographolide
A technology of andrographolide and crystallization, applied in the field of medicine, can solve problems such as poor water solubility of andrographolide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] Preparation of andrographolide raw material:
[0057] The raw material of andrographolide was prepared according to the method of Example 1 in CN102382083A (Chinese Patent Application No. 201010268676.0). After determination, its content is 98.1% (HPLC), and powder X-ray diffraction is determined. In the powder X-ray diffraction pattern represented by 2θ angle, there is no Diffraction peaks.
Embodiment 1
[0058] Embodiment 1: preparation andrographolide crystallization
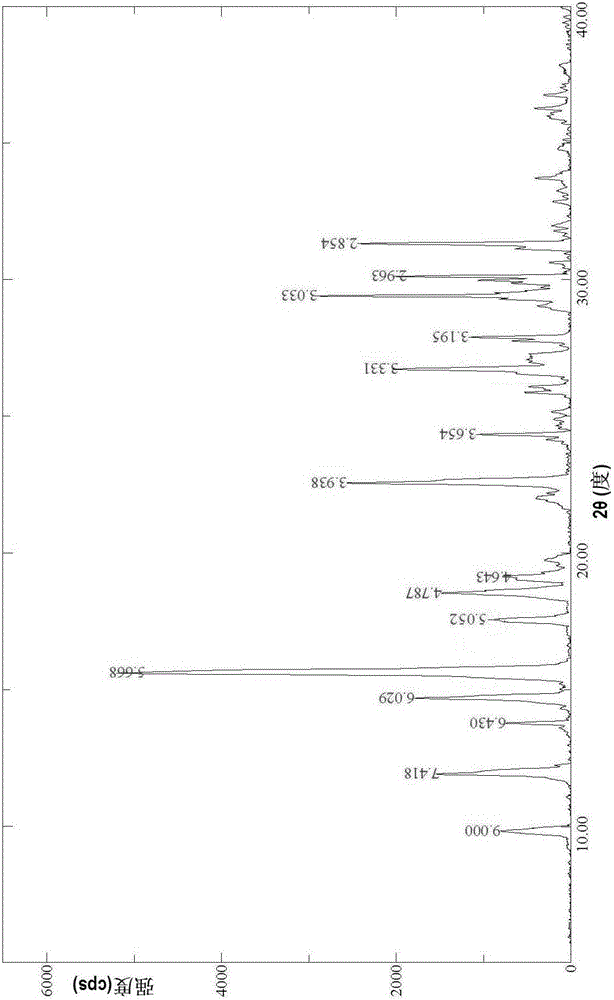
[0059] Place 50 g of the andrographolide raw material prepared according to the method of Example 1 in CN102382083A above in 1000 ml of 98% ethanol, heat to boiling and stir to dissolve the andrographolide, add 50 ml of n-butanol while hot, and stir evenly , stand still, let cool to room temperature to separate out the precipitate, filter out the precipitate, wash with absolute ethanol, then wash with water, and dry with air heat at 60-65°C, to obtain that, the yield is 96.3%, and the content is 99.2% (HPLC) , determined by powder X-ray diffraction, the results are as follows figure 1 .
[0060] The results of powder X-ray diffraction show that in the powder X-ray diffraction pattern represented by 2θ angles, the crystals have There are diffraction peaks at °; particularly, in the powder X-ray diffraction pattern represented by 2θ angles, the crystals are at about 9.82°, about 11.92°, about 14.68°, about 15...
Embodiment 2
[0063] Embodiment 2: preparation andrographolide crystallization
[0064] The commercially available andrographolide bulk drug (National Medicine Accreditation H51023720, produced by Chengdu Tiantaishan Pharmaceutical Co., Ltd., the test report meets the requirements of the 2010 edition Chinese Pharmacopoeia Part One "andrographolide" variety, which is about 9.82°, about 11.92°, About 15.62 °, no diffraction peak at about 22.56 °) 50g is placed in 95% ethanol of 1000ml, heated to boiling and under stirring so that andrographolide dissolves, add 20ml n-butanol while hot, stir evenly, leave standstill, Let it cool to room temperature to precipitate the precipitate, filter the precipitate, wash with absolute ethanol, then wash with water, and dry in vacuum at 60-65°C to obtain that, the yield is 96.7%, the content is 99.3% (HPLC), and the powder X-ray is determined Diffraction, as a result, in the powder X-ray diffraction pattern represented by 2θ angle, the crystal is in terms...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com