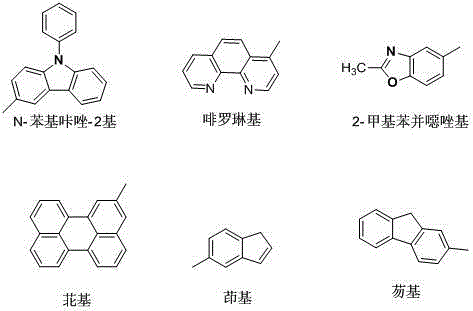
Benzanthracene organic light-emitting material and preparation method thereof
A technology of luminescent materials, benzanthracene, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
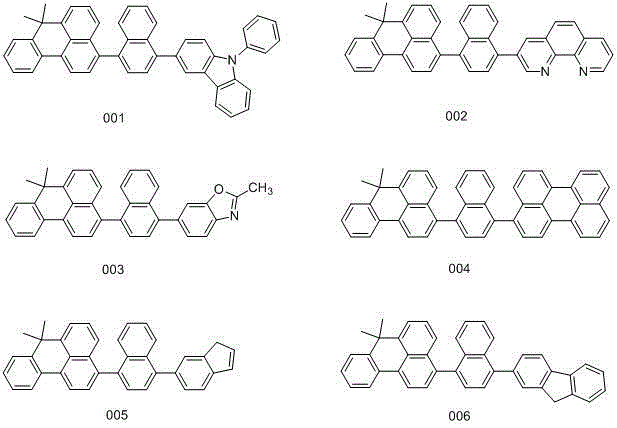
[0019] Embodiment 1: the synthesis of compound 001
[0020] The specific synthetic route is shown in the following formula:
[0021]
[0022] 22.47g (50mmol) of 3-(4-bromonaphthyl)-7,7-dimethyl-7H-benzanthracene, 21.69g (75mmol) of N-phenylcarbazolylboronic acid, 10.60g (100mmol) of sodium carbonate ), 250ml of tetrahydrofuran and 125ml of water were added to a three-necked flask, degassed, added 0.58g (0.5mmol) of tetrakis(triphenylphosphine) palladium, heated to 70°C, reacted for 15 hours, cooled to room temperature, and after the solid precipitated, suction filtered, The filter cake was washed with water, ethanol and ether, and then dried to obtain 28.45 g of asymmetric benzanthracene derivatives, with a yield of over 93% and an HPLC purity of over 98%. Mass Spectrum: Calcd. 611.77; Tested 611.74. Elemental Analysis: Calcd: C: 92.27%; H: 5.44%; N: 2.29%; Tested: C: 92.25%; H: 5.45%; N: 2.30%.
[0023]
Embodiment 2
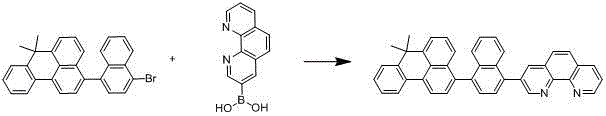
[0024] Embodiment 2: the synthesis of compound 002
[0025] The specific synthetic route is shown in the following formula:
[0026]
[0027] 22.47g (50mmol) of 3-(4-bromonaphthyl)-7,7-dimethyl-7H-benzanthracene, 17.92g (80mmol) of phenanthrene boronic acid, 12.19g (115mmol) of sodium carbonate, 250ml of tetrahydrofuran and Add 125ml of water into a three-necked flask, degas, add tetrakis(triphenylphosphine)palladium 0.69g (0.6mmol), raise the temperature to 80°C, react for 17 hours, cool to room temperature, after the solid precipitates, suction filter, the filter cake is washed with water, After washing with ethanol and ether, 25.51 g of asymmetric benzanthracene derivatives were obtained by drying, with a yield of more than 93% and a purity of more than 98% by HPLC. Mass spectrum: calculated value 548.67; found value 548.66. Elemental analysis: calculated value C: 89.75%; H: 5.14%; N: 5.11%; tested value C: 89.73%; H: 5.15%; N: 5.12%.
[0028]
Embodiment 3
[0029] Embodiment 3: the synthesis of compound 003
[0030] The specific synthetic route is shown in the following formula:
[0031]
[0032] 22.47g (50mmol) of 3-(4-bromonaphthyl)-7,7-dimethyl-7H-benzanthracene, 15.04g (85mmol) of 2-methylbenzoxazolylboronic acid, and 13.78g of sodium carbonate (130mmol), 250ml of tetrahydrofuran and 125ml of water were added to a three-necked flask, degassed, added 0.81g (0.7mmol) of tetrakis(triphenylphosphine)palladium, heated to 85°C, reacted for 19 hours, cooled to room temperature, and after the solid was precipitated, pumped After filtering, the filter cake was washed with water, ethanol and ether, and then dried to obtain 23.14 g of asymmetric benzanthracene derivatives, with a yield of more than 93% and an HPLC purity of more than 98%. Mass spectrum: Calculated value is 501.62; found value is 501.64. Elemental analysis: calculated value C: 88.59%; H: 5.43%; N: 2.79%; O: 3.19%; tested value C: 88.59%; H: 5.42%; N: 2.78%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com