Adenovirus vector and production method for same
A production method, the technology of adenovirus, applied in the field of bioengineering, can solve the problems of inability to meet the needs of adenovirus gene cloning production, high-throughput adenovirus production, and unreliability, so as to improve recombination efficiency, reduce costs, and shorten time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
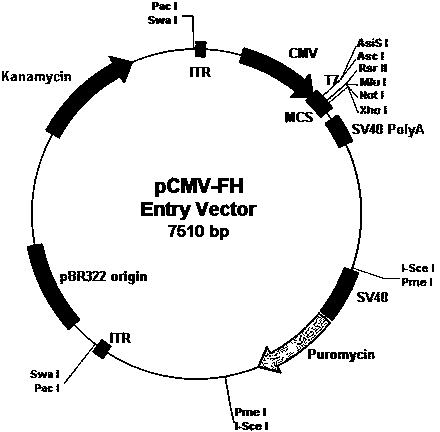
[0040] Example 1 .shuttle vector pCMV-FH
[0041] The shuttle carrier pCMV-FH of the present invention is transformed on the basis of pCMV-Shuttle by conventional molecular biology techniques, and the specific steps are:
[0042] (1). Shorten the left and right homology arms. The recombinant sequences on the left and right sides of the vector were shortened to 800 bases. The right homology arm of 1243-2043 bases and the left homology arm of 3545-4428 bases of the original plasmid were retained. Primers were designed using general molecular biology primer design methods. A PmeI restriction site was added at both ends of the PCR primers. After PCR amplification, the PCR products were self-ligated, then transformed into competent cells, extracted and sequenced.
[0043] (2). Add a Puromycin gene at the PmeI restriction site. The Puromycin gene, SV40 promoter and BGH PolyA signal were used for gene synthesis in vitro. After the synthetic product was digested with PmeI, it ...
Embodiment 2
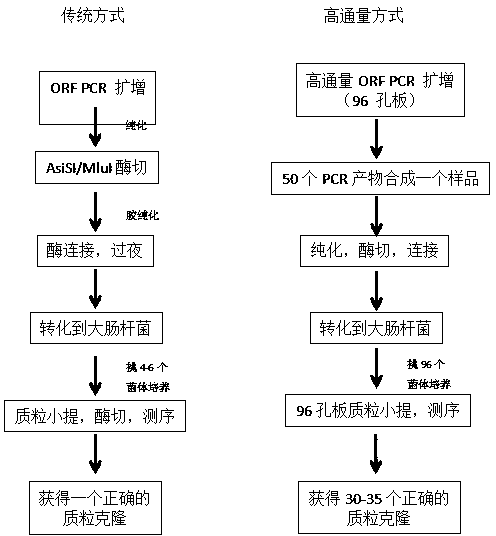
[0046] Example 2. Construction of high-throughput shuttle clones
[0047] It is transformed by conventional molecular biology techniques, and the specific steps are:
[0048] (1). The full-length DNA template for PCR (the full-length human gene comes from the RefSeq database at http: / / www.ncbi.nlm.nih.gov / RefSeq / ) is first arranged on a 96-well PCR plate. 30,000 ORF primers (purchased from Eurofins / MWG / Operon Biological Company in the United States, see the attached Figure 5 ) are also designed in the order of the DNA template.
[0049] (2). 15,000 existing clones (12,000 from the National Health Service of the United States and 3,000 from Qingdao Macleay Biotechnology Co., Ltd.) were amplified in a 96-well plate format. To reduce mutations during PCR, each gene was amplified for only 15 rounds, and a high-fidelity polymerase was used. (Example 3 takes the NM_000632 gene as an example to describe in detail the human whole genome gene PCR)
Embodiment 3
[0049] (2). 15,000 existing clones (12,000 from the National Health Service of the United States and 3,000 from Qingdao Macleay Biotechnology Co., Ltd.) were amplified in a 96-well plate format. To reduce mutations during PCR, each gene was amplified for only 15 rounds, and a high-fidelity polymerase was used. (Example 3 takes the NM_000632 gene as an example to describe in detail the human whole genome gene PCR)
[0050] (3). According to the size of the ORF, take 2 microliters of each PCR product, combine 50 genes of similar size into a test tube, and then purify.
[0051] (4). Digest according to the enzyme cut point, separate and purify on agarose gel.
[0052] (5). Linked to the shuttle vector pCMV-FH and transformed into competent cells.
[0053] (6). Pick 96 bacterial cells, culture them in 96-well deep-well plates, extract and sequence the plasmids.
[0054] (7). The sequence at the 5' end is compared with the sequence of the original 50 ORFs.
[0055] (8). The 5'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com