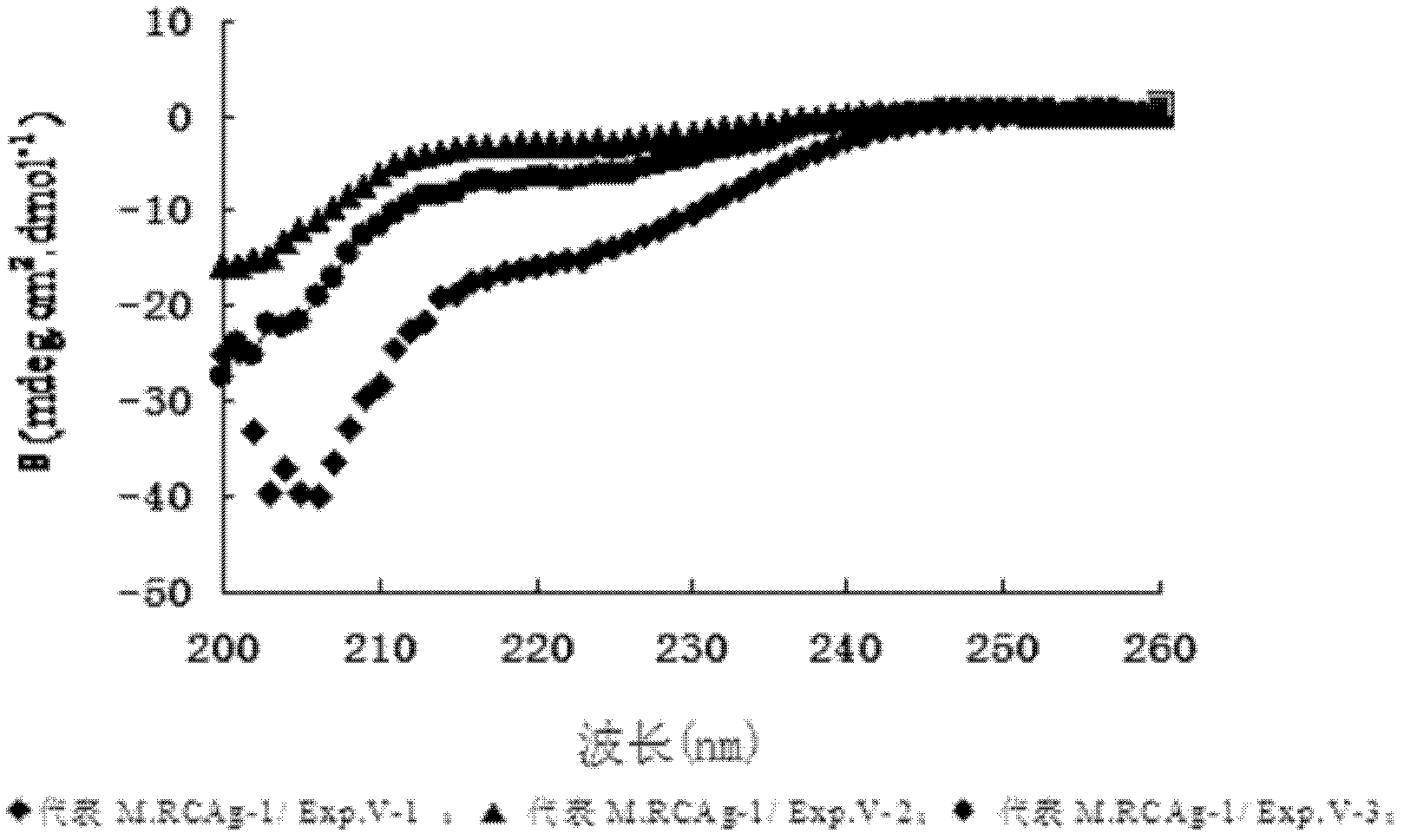Fusion protein, use thereof, and anti-malarial vaccine and antibody thereof
A fusion protein and anti-malarial technology, applied in the field of biomedicine, can solve the problem of difficulty in obtaining artificial polyepitope antigens, and achieve the effects of good development prospects, stable physical and chemical properties, and inhibition of in vitro growth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
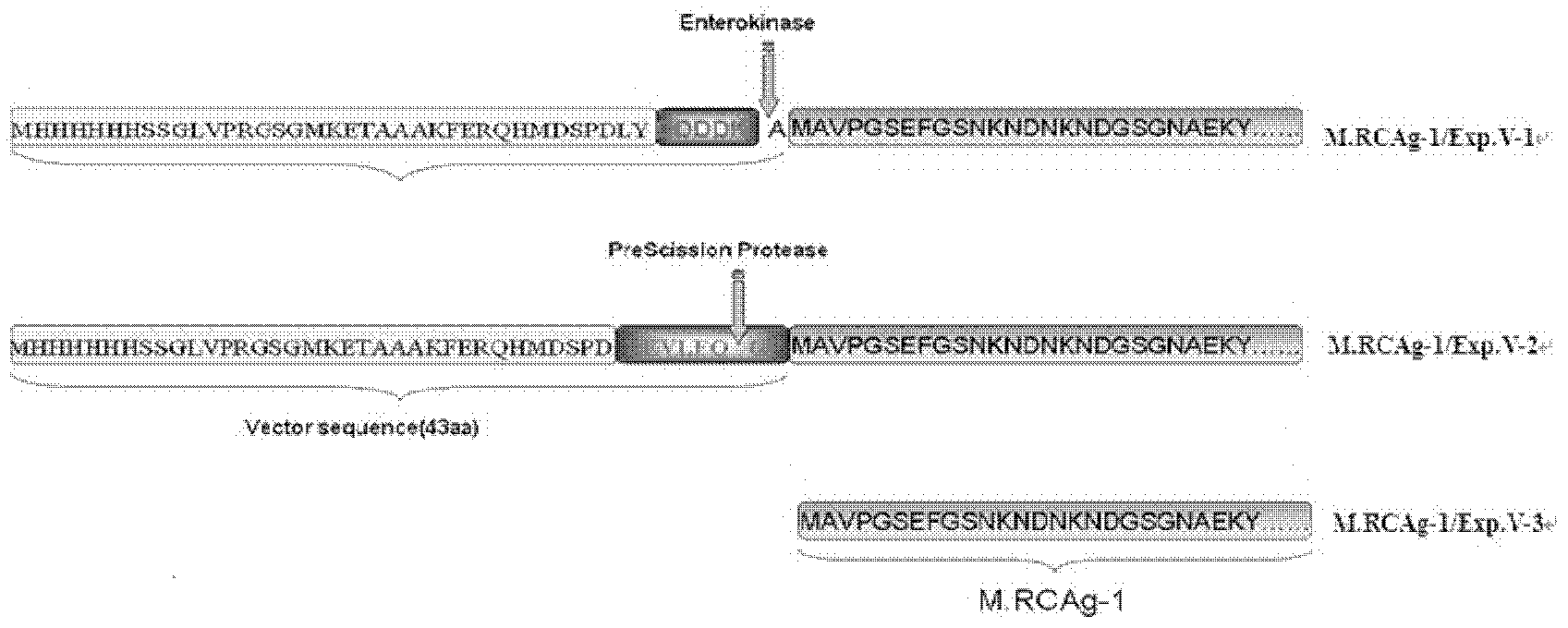
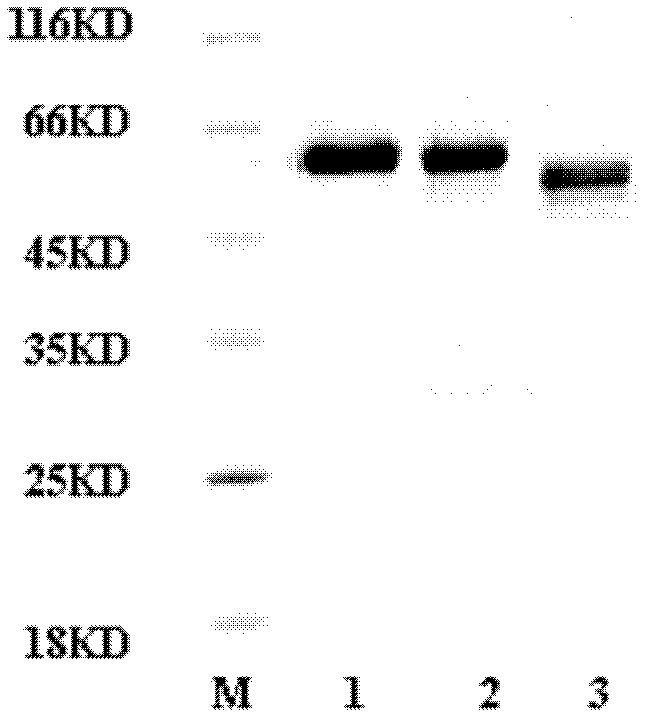
[0056] According to one embodiment of the present invention, the M.RCAg-1 gene is cloned into the prokaryotic expression vector pDS-ex containing different carrier tags or no carrier tag, expressed and purified to obtain M.RCAg-1 / Exp.V-1 , M.RCAg-1 / Exp.V-2, M.RCAg-1 / Exp.V-3 three kinds of multi-epitope proteins, the amino acid sequences of which are respectively as SEQ ID No.: 1, SEQ ID No.: 3 and Shown in SEQ ID No.: 5; its DNA sequences are shown in SEQ ID No.: 2, SEQ ID No.: 4 and SEQ ID No.: 6, respectively.
[0057] figure 1 Shows the amino acid sequence comparison schematic diagram of M.RCAg-1 / Exp.V-1, M.RCAg-1 / Exp.V-2 and M.RCAg-1 / Exp.V-3 three kinds of multi-epitope proteins, wherein M.RCAg-1 / Exp.V-1 is a multi-epitopic protein with 43 carrier amino acids fused at the N-terminus with an enterokinase digestion recognition site, and M.RCAg-1 / Exp.V-2 is in A multi-epitope protein with 43 carrier amino acids fused to the N-terminus with a PreScission Protease cleavage re...
Embodiment 1
[0063] Embodiment 1 constructs VR1012-312 plasmid and pDS-ex plasmid
[0064] According to the method disclosed in Chinese Patent Application No. 200410080982.6, VR1012-312 plasmid and pDS-ex plasmid were constructed. details as follows:
[0065] According to the method disclosed in Chinese Patent Application No.200410080982.6, the gene fragment m.rcag-1 (that is, the ES312 gene fragment disclosed in Chinese Patent Application No.200410080982.6) was obtained, and the resulting gene fragment m.rcag-1 was connected to pDS- On the ex plasmid (according to the method disclosed in Chinese Patent Application No.200410080982.6, the prokaryotic expression vector pET-30a (+) (obtained from Novagen) was replaced by Bgl II with the Not I and Eag I restriction sites by PCR technology, Kpn I is moved forward, and EcoRV, Bgl II, and Nsp V restriction sites are removed, thereby obtaining the pDS-ex plasmid), its DNA sequence is shown in SEQ ID No.: 10, and its physical map is Figure 7 sho...
Embodiment 2
[0066] Embodiment 2 obtains M.RCAg-1 / Exp.V-1 protein
[0067] 1. The VR1012-312 plasmid and pDS-ex plasmid obtained in Example 1 were subjected to EcoR I and BglII double enzyme digestion respectively, so that m.rcag-1 was connected into the pDS-ex vector, thereby constructing the recombinant plasmid M.RCAg- 1 / pDS-ex.
[0068] The specific steps are as follows:
[0069] 1) The restriction enzyme digestion system of VR1012-312 plasmid and pDS-ex plasmid (taking EcoR I and Bgl II as examples) is as follows:
[0070]
[0071] Digest at 37°C for 2 hours, identify by agarose gel electrophoresis, and recover with a DNA fragment recovery kit to obtain gene fragment m.rcag-1 and pDS-ex plasmid fragment.
[0072] 2) Dephosphorylation of the recovered gene fragment m.rcag-1 and pDS-ex plasmid fragment
[0073] Concrete reaction system is as follows:
[0074]
[0075] 3) In the ligation reaction of the recovered gene fragment m.rcag-1 and the pDS-ex plasmid fragment, the molar ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com