Preparation method of perindopril arginine salt of gamma-crystal form
A technology of perindopril arginine salt and perindopril, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of prolonging the drying time, unfavorable suction filtration, and reducing yield, etc. problem, to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Preparation of Perindopril
[0024] Dissolve 60.0g of perindopril tert-butylamine salt in 240mL of water, add 240mL of dichloromethane, stir at 25-30℃, then add 2.0mol / L hydrochloric acid dropwise to the above solution to adjust the pH of the water phase It is 3.8-4.4, finally separated, extracted with dichloromethane, and concentrated under reduced pressure to obtain perindopril.
Embodiment 2
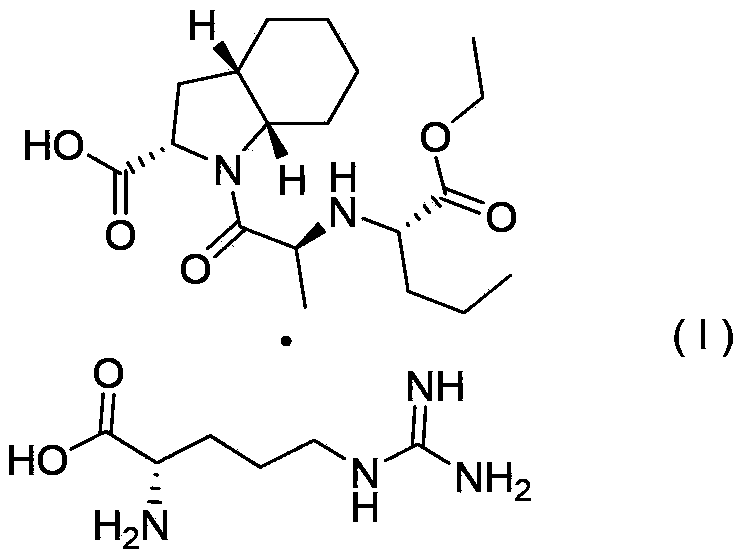
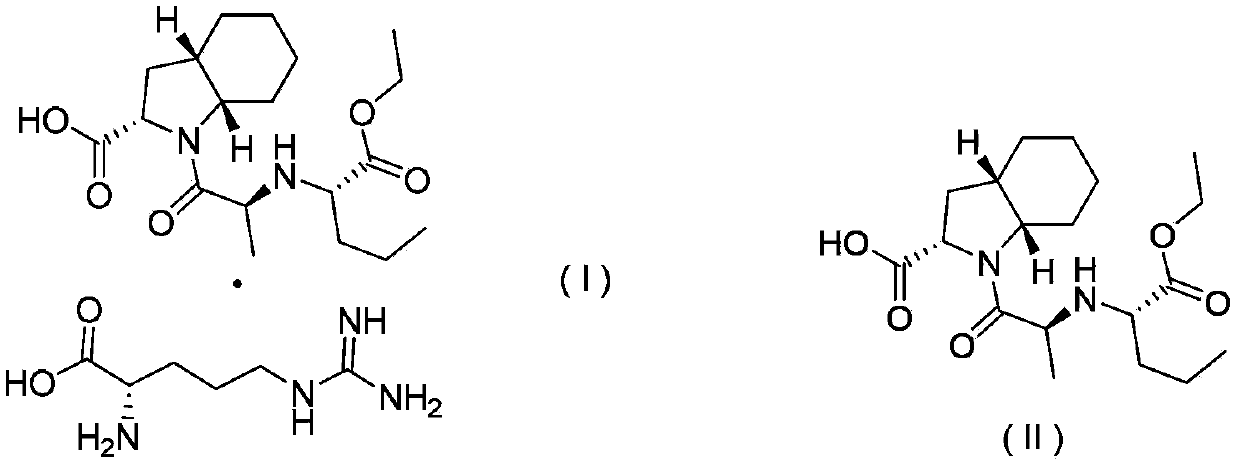
[0025] Example 2. Preparation of perindopril arginine salt (I) in γ crystal form
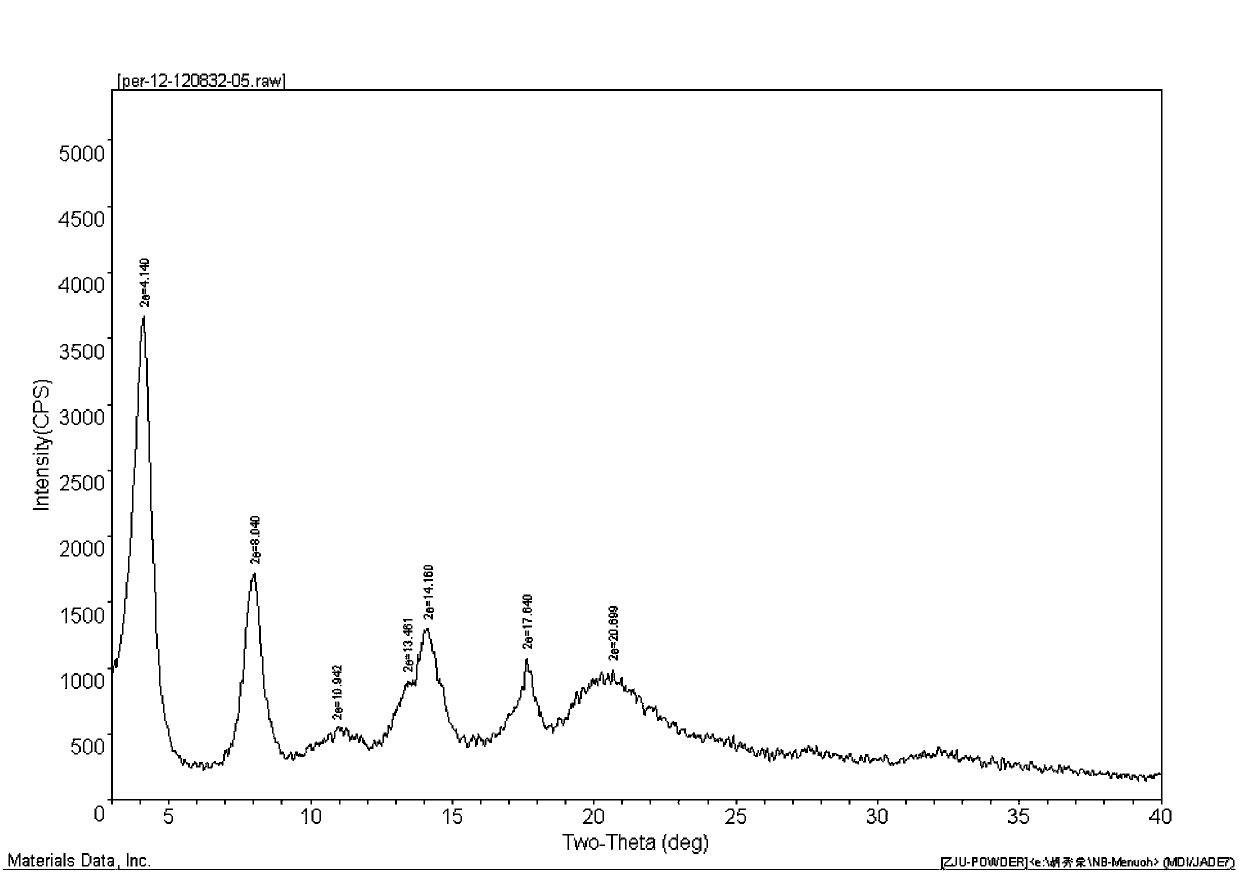
[0026] Add 5.0g (0.0136mol) of perindopril, 2.13g (0.0122mol) of L-arginine, and 10mL of water to a 150mL four-neck flask, and stir until it is clear. Then add 100 mL of cyclohexane to the above solution, start to heat up and reflux to separate water, no more water enters in the water separator, water separation is completed when the solvent is no longer stratified, and the temperature begins to drop. After cooling down to room temperature, start suction filtration. After suction filtration, the white powder solid (weight 6.36g, yield 95.9%, HPLC content: 99.98%) obtained after drying the filter cake is the final product of perindopril γ crystal form Arginine salt, X-ray powder diffraction pattern see attached figure 1 It can be seen that the product is perindopril arginine salt of γ crystal form. Cyclohexane in the filtrate obtained by suction filtration can be recovered by simple distillation. ...
Embodiment 3
[0027] Example 3. Preparation of perindopril arginine salt (I) in γ crystal form
[0028] Add 5.0g (0.0136mol) of perindopril, 1.66g (0.0095mol) of L-arginine, and 5mL of water to a 150mL four-necked flask, and stir until it is clear. Then, 50 mL of cyclohexane was added to the above solution, and the temperature was increased to reflux to separate water. There is no more water in the water separator, the water separation is completed when the solvent is no longer stratified, and the temperature starts to drop. The temperature is lowered to 30°C and suction filtration is started. The white powder solid (weight 4.80g, yield 92.8%, HPLC content: 99.95%) obtained after suction filtration and drying of the filter cake is the final product of perindopril γ crystal form Arginine salt, X-ray powder diffraction pattern and attached figure 1 Consistent. Cyclohexane in the filtrate obtained by suction filtration can be recovered by simple distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com