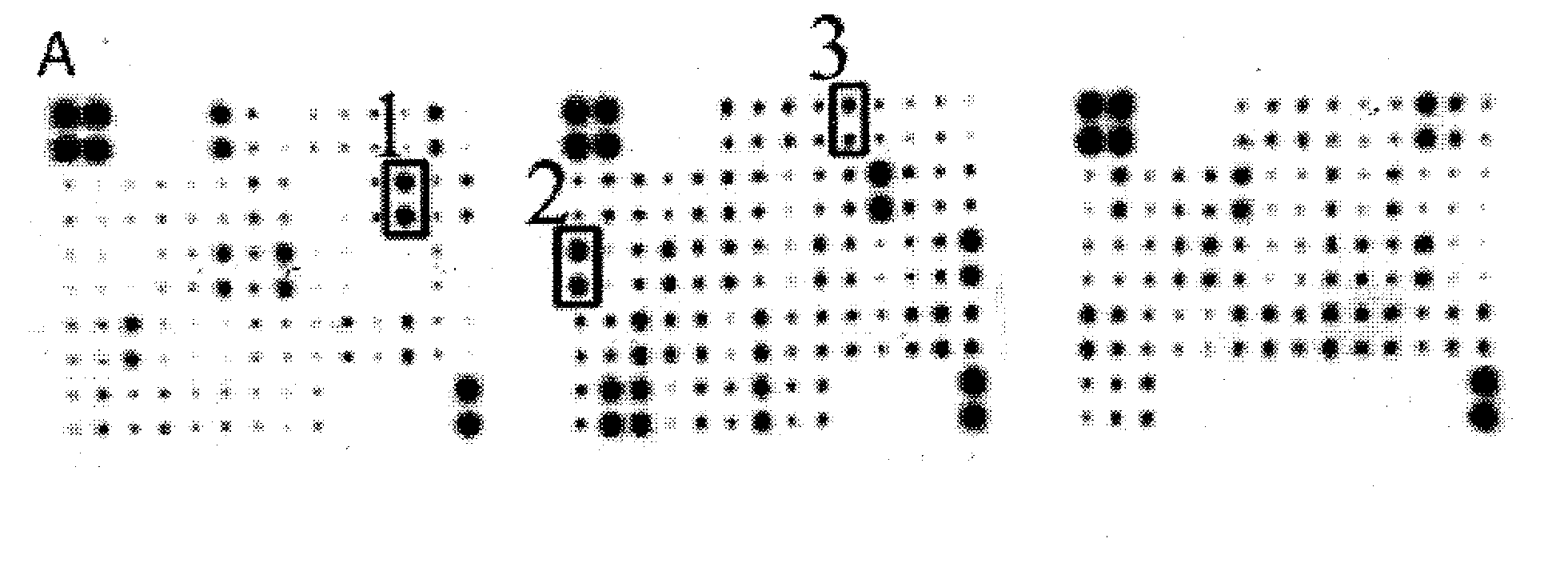Preparation method of immunochromatography test paper for detecting premature rupture of fetal membranes
A technology of immunochromatographic test strips and premature rupture of membranes, which is applied in the direction of measuring devices, analysis materials, instruments, etc., can solve the problems of easy misdiagnosis, unsatisfactory sensitivity, specificity and accuracy, and inconvenience for obstetric patients. Effects of Sensitivity and Specificity Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
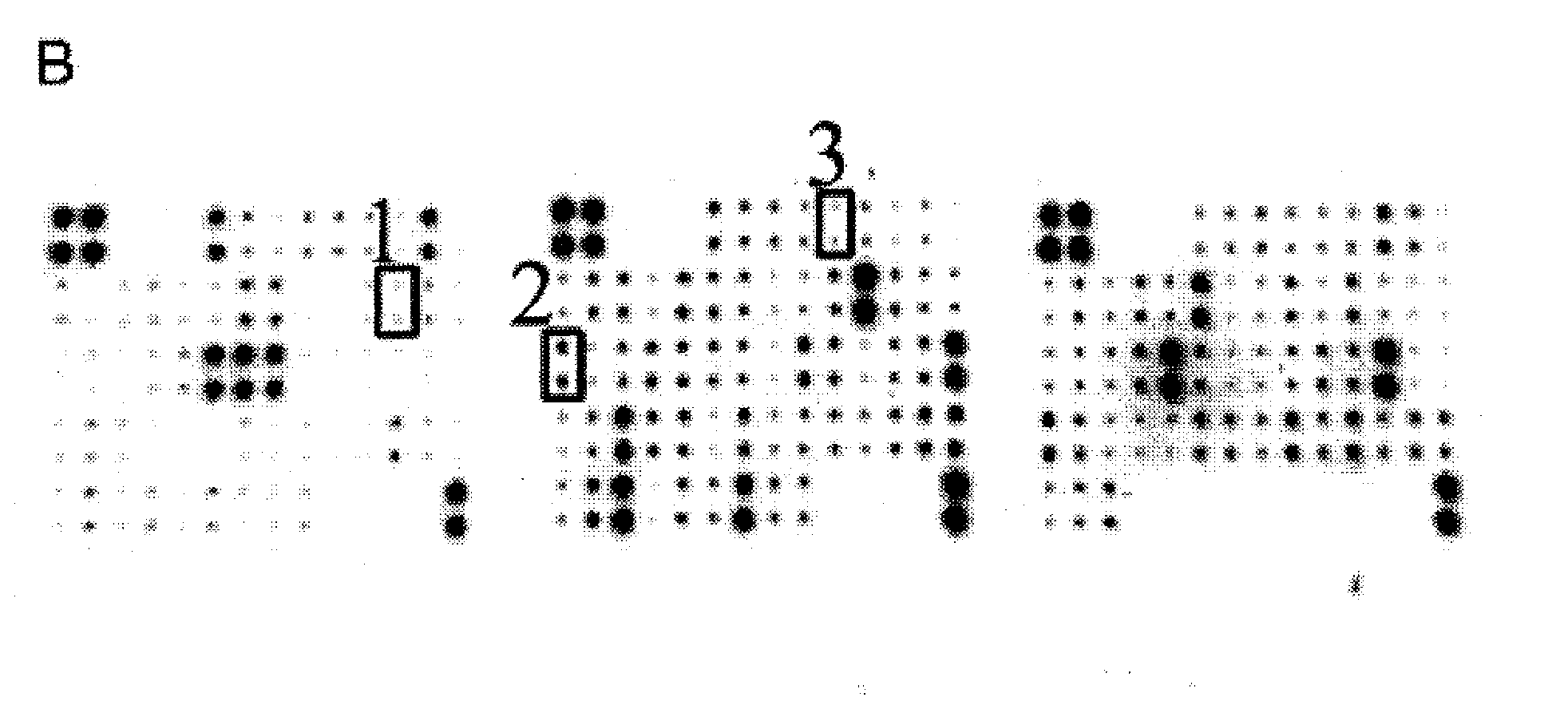
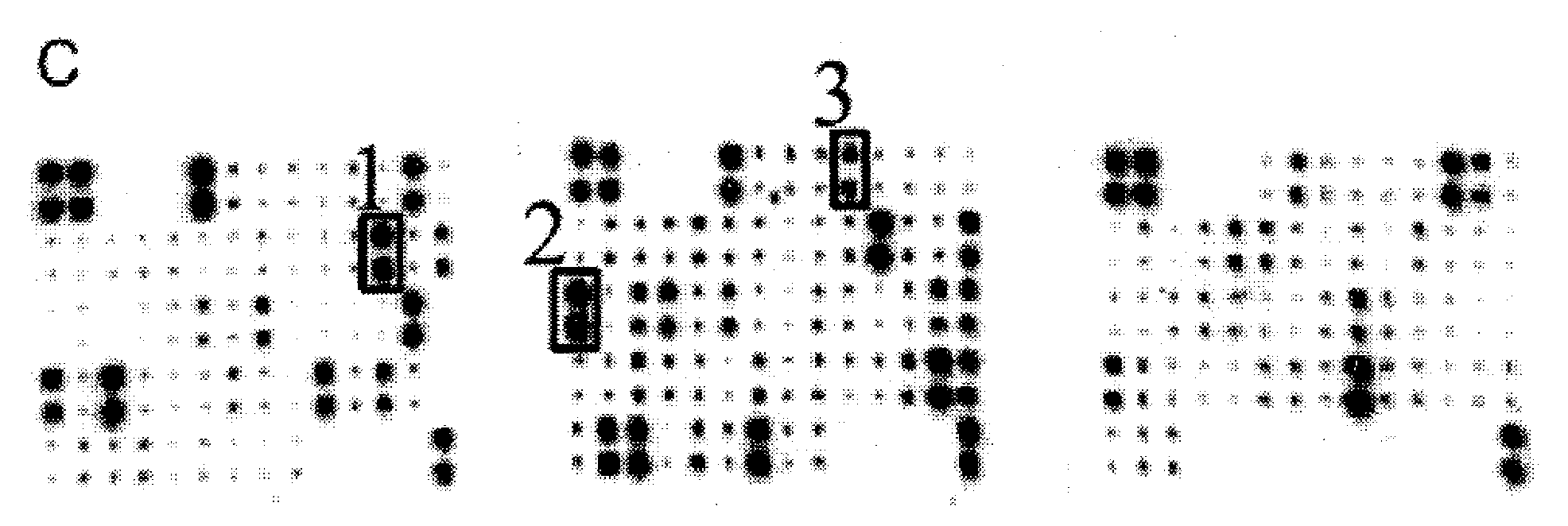
[0052] Premature rupture of membranes group: 110 pregnant women with premature rupture of membranes (including 80 cases of full-term premature rupture of membranes and 30 cases of preterm premature rupture of membranes);
[0053] Healthy control group: 110 pregnant women with intact membranes before delivery;
[0054] Amniotic fluid sample group: 30 pregnant women with intact membranes undergoing cesarean section.
[0055] Clinical screening criteria for experimental subjects:
[0056] Premature rupture of membranes group—(i) Leakage of amniotic fluid was observed before labor;
[0057] (ii) The pH test paper test of the vaginal secretion sample is positive;
[0058] (iii) Positive detection of fern leaf crystals.
[0059] Healthy control group—(i) Amniotic fluid leakage was not observed before labor;
[0060] (ii) The pH test paper test of the vaginal secretion sample is negative;
[0061] (iii) Negative test for fern leaf ...
Embodiment 2
[0180] The control example 1-20 immunochromatographic test papers prepared by different methods were used to detect the clinical samples of vaginal secretions of the same pregnant women respectively, including premature rupture of membranes group and healthy control group, wherein the premature rupture of membranes group was 240 cases, 260 cases in the healthy control group, and the test subjects were all clinically diagnosed and screened using the method in Example 1.
[0181] Sample collection: Pregnant women were taken in the bladder lithotomy position (lying in the supine position, with the lower limbs flexed separately, exposing the vulva), after peeping through the vagina, a disposable sterile cotton swab was inserted into the posterior fornix of the vagina, and rotated 5 times to sample. Insert the cotton swab head into 1mL sample diluent (phosphate buffer system), rotate 5 times, squeeze and rotate 2 times against the tube wall to obtain the sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com