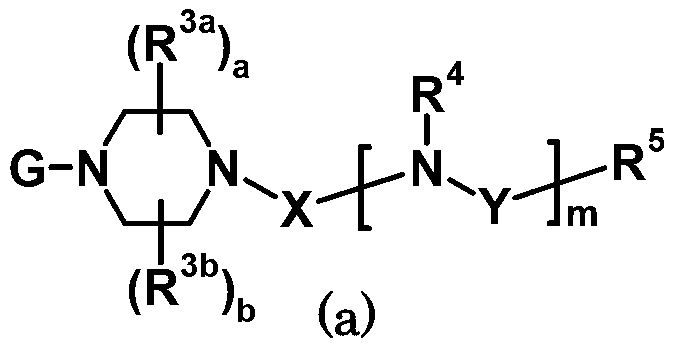
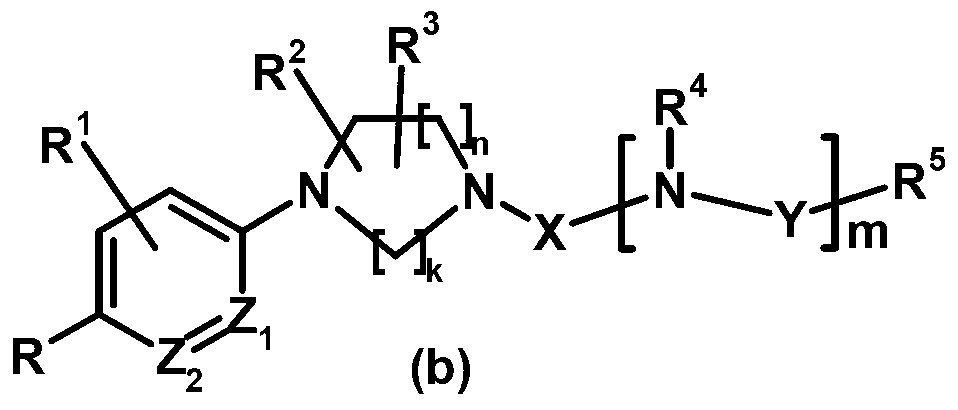
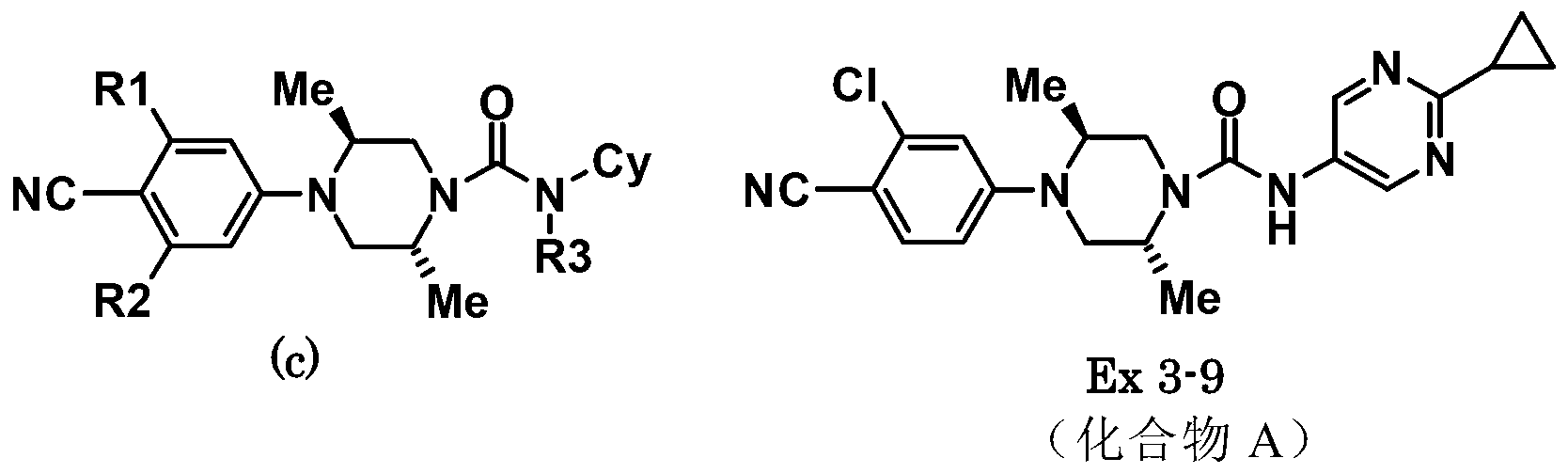
Antagonist for mutated androgen receptor
A technology of resistance and castration resistance, which can be used in anti-tumor drugs, endocrine system diseases, urinary system diseases, etc., and can solve problems such as no disclosure or revelation, and ineffective AR antagonists.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0183] To a mixture of potassium tert-butoxide (505 mg) and THF (5 ml) was added dropwise ethanol (0.264 ml) under ice-cooling, followed by stirring at room temperature for 30 minutes. The reaction solution was ice-cooled, a mixture of 4-[(2S,5R)-2,5-dimethylpiperazin-1-yl]-2-fluorobenzonitrile (350mg) and THF (3ml) was added dropwise, and Stir at room temperature for 16 hours. Ethyl acetate (20 ml) and water (10 ml) were added to the reaction solution for liquid separation, and the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (chloroform / methanol=100 / 0-96 / 4) to obtain 4-[(2S,5R)-2,5-dimethylpiperazin-1-yl]-2- Ethoxybenzonitrile 315mg (81%). ESI+: 260
preparation example 2
[0185] To a mixture of piperazin-2-one (5.06g) and DMF (50ml) was added triethylamine (8ml) and 2-chloro-4-fluorobenzonitrile (8.1g) and stirred at 80°C for 1 day . Water was added to the reaction solution, and the precipitated solid was filtered, and washed with water and diisopropyl ether to obtain 11.2 g (94%) of 2-chloro-4-(3-oxopiperazin-1-yl)benzonitrile. EI+: 235
preparation example 3
[0187] To a mixture of 2-chloro-4-(3-oxopiperazin-1-yl)benzonitrile (11.2g) and DMF (160ml) was added sodium hydride (2.2g, 55% liquid paraffin dispersion), in After stirring at room temperature for 10 minutes, benzyl bromide (6 ml) was added and stirred at room temperature for 1 hour. Add water to the reaction solution, filter the precipitated solid, then wash with water and diisopropyl ether to obtain 12.74 g of 4-(4-benzyl-3-oxopiperazin-1-yl)-2-chlorobenzonitrile (82%). EI+: 325
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com