Carboxylated carbon nanotube catalyst carrier as well as preparation method and application thereof
A catalyst carrier, carboxylated carbon technology, used in catalyst carriers, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as application difficulties, achieve a high degree of carboxylation, improve inertness and hydrophobicity, and surface containing Oxygen rich effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 (preparation of carboxylated CNT)
[0036] The preparation process of CNT-95 is: take 3g CNT and add to 150mL concentrated HNO with a volume ratio of 3:2 3 and concentrated H 2 SO 4 The mixture was then transferred to a Erlenmeyer flask, refluxed in a water bath at 95°C for 100 minutes, then washed with deionized water in a vacuum filtration device until nearly neutral, and finally dried in air at 110°C for 16 hours.
[0037] The preparation process of CNT-80 is as follows: take 4.5g CNT and add to 240mL concentrated HNO with a volume ratio of 1:3 3 and concentrated H 2 SO 4 The mixed solution was then transferred to a conical flask, refluxed in a water bath at 80°C for 12 hours, then washed with deionized water in a vacuum filtration device until nearly neutral, and finally dried in air at 110°C for 16 hours.
[0038] Through the experiments of carboxylated CNTs under different conditions in this example, two kinds of carboxylated CNTs, namely CNT-80 an...
Embodiment 2
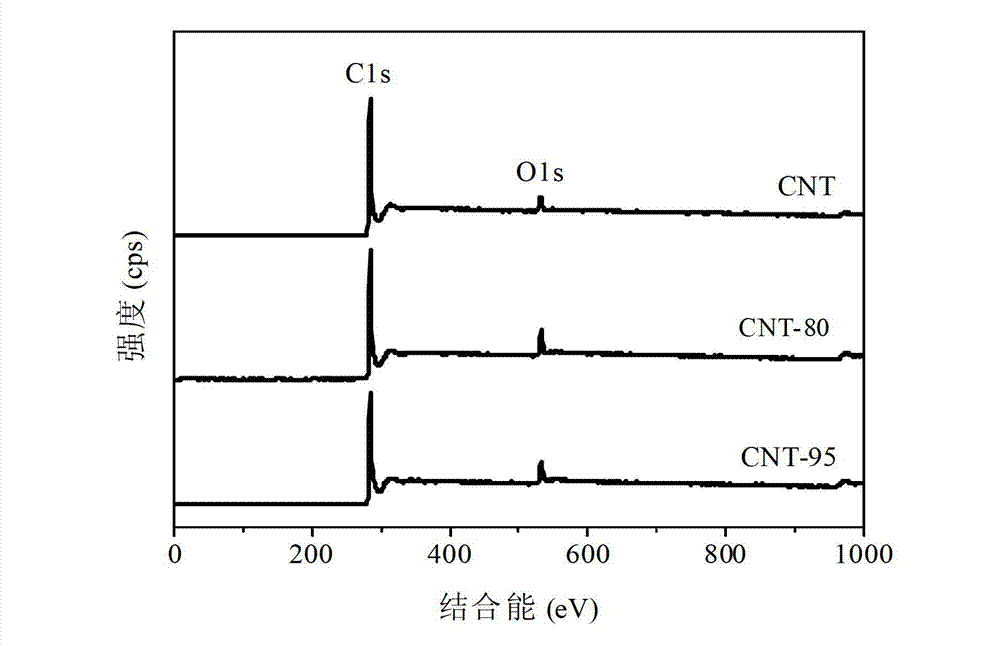
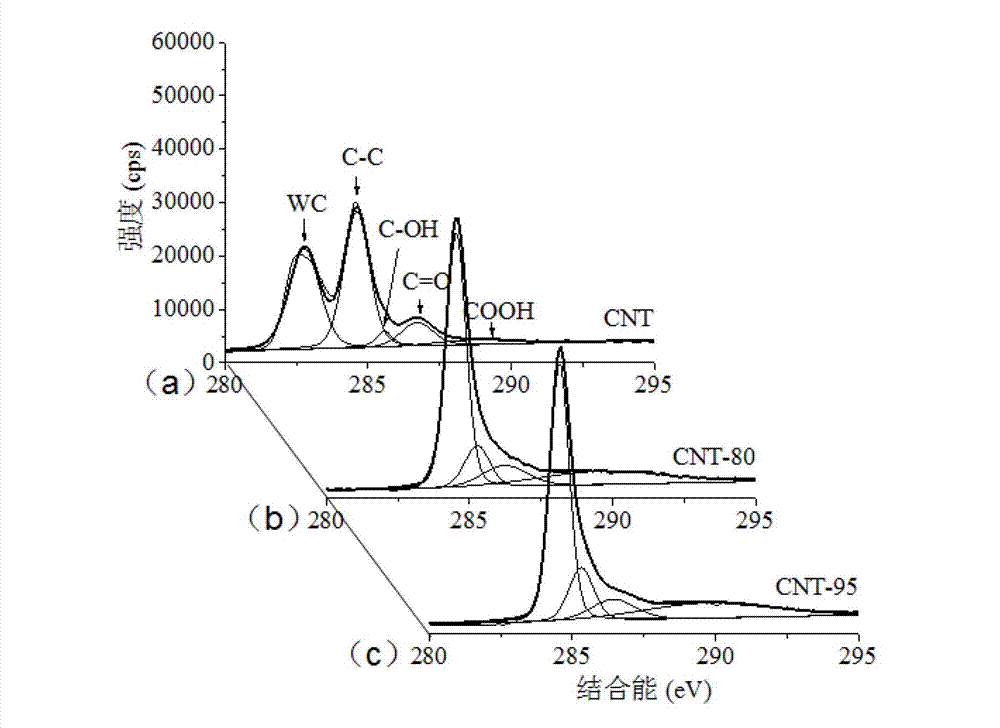
[0039] Example 2 (XPS characterization of carboxylated CNT)
[0040] The XPS tests of untreated CNTs and two carboxylated CNTs were carried out on a multifunctional X-ray photoelectron spectrometer (model Axis Ultra DLD), and the vacuum degree of the analysis chamber was about 5×10 -9 torr, the X light source used is a monochromatic Al Kα source (Mono AlKα), the energy is 1486.6eV, 10mA×15KV, and the beam spot size is 700×300μm; The energy is 40eV, and the number of scans is 1 time. Before the result analysis, the C1s of each sample was divided into peaks, and then the binding energy of C1s at the lowest energy end was calibrated to 284.6eV, and each element was corrected according to the shift of C1s peak position.
[0041] Through the XPS analysis of untreated CNT and two carboxylated CNTs in this example, after acid modification at 95°C, the tube wall of CNT is oxidized, the port is opened, and the surface is formed in addition to groups such as -COOH and -OH. Will genera...
Embodiment 3
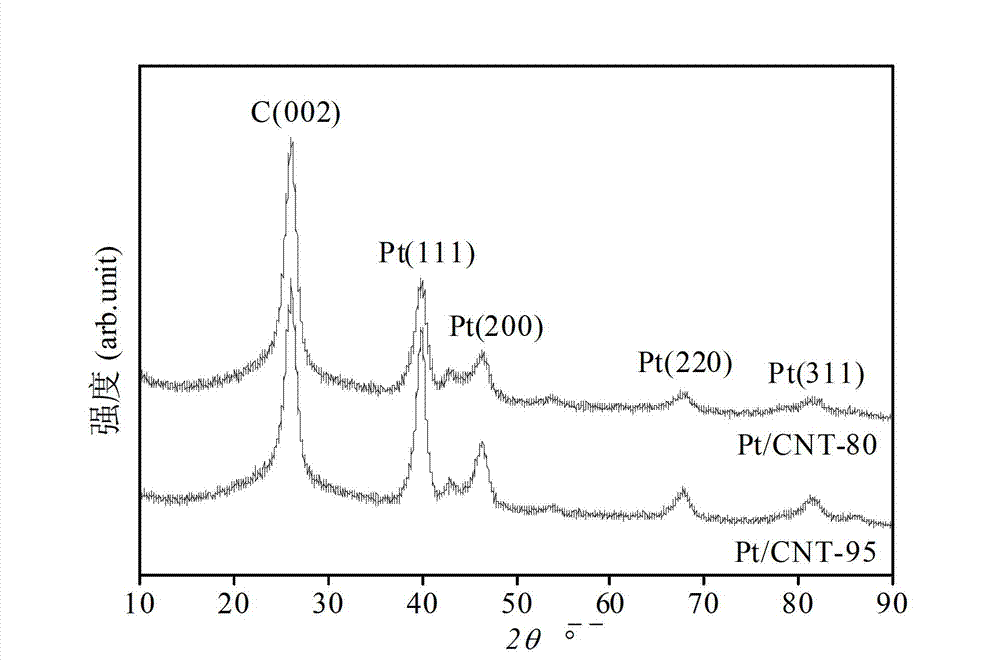
[0042] Embodiment 3 (the application of carrier: the preparation of Pt / CNT catalyst)
[0043] Pt / CNT-80 and Pt / CNT-95 catalysts were prepared by impregnation-precipitation method, 0.675gH 2 PtCl 6 ·6H 2 O was dissolved in 25mL of ethanol to prepare a 0.0193mol / L ethanol solution of chloroplatinic acid. According to the theoretical platinum load of 10%, 2.5g of CNT-80 and 3.5g of CNT-95 were placed in the chloroplatinic acid solution respectively. Mix well in ethanol solution, adjust the pH value to 7.5 with 0.2mol / L NaOH solution, add excess 0.1mol / L HCHO solution, reduce at 80°C for 2h, then wash with deionized water until the filtrate is free of Cl - (AgNO 3 solution test), and finally dried in a vacuum desiccator at 80°C for 12 hours. The Pt / CNT catalysts prepared with CNT-80 and CNT-95 are denoted as Pt / CNT-80 and Pt / CNT-95, respectively.
[0044] Through this example, Pt / CNT-80 and Pt / CNT-95 catalysts were obtained by impregnation-precipitation method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| internal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com