Supercritical carbon dioxide dispersion polymerization stabilizer as well as preparation method and using method thereof
A technology of carbon dioxide and dispersion polymerization, applied in the production of bulk chemicals, etc., can solve the problem of difficult to meet requirements, high cost of stabilizers (such as fluorine-containing stabilizers and most siloxane-based stabilizers, toxic working pressure of stabilizers and other issues to achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
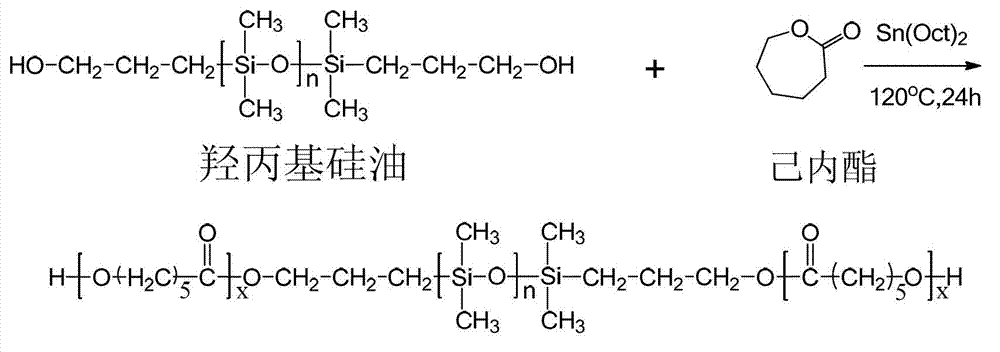
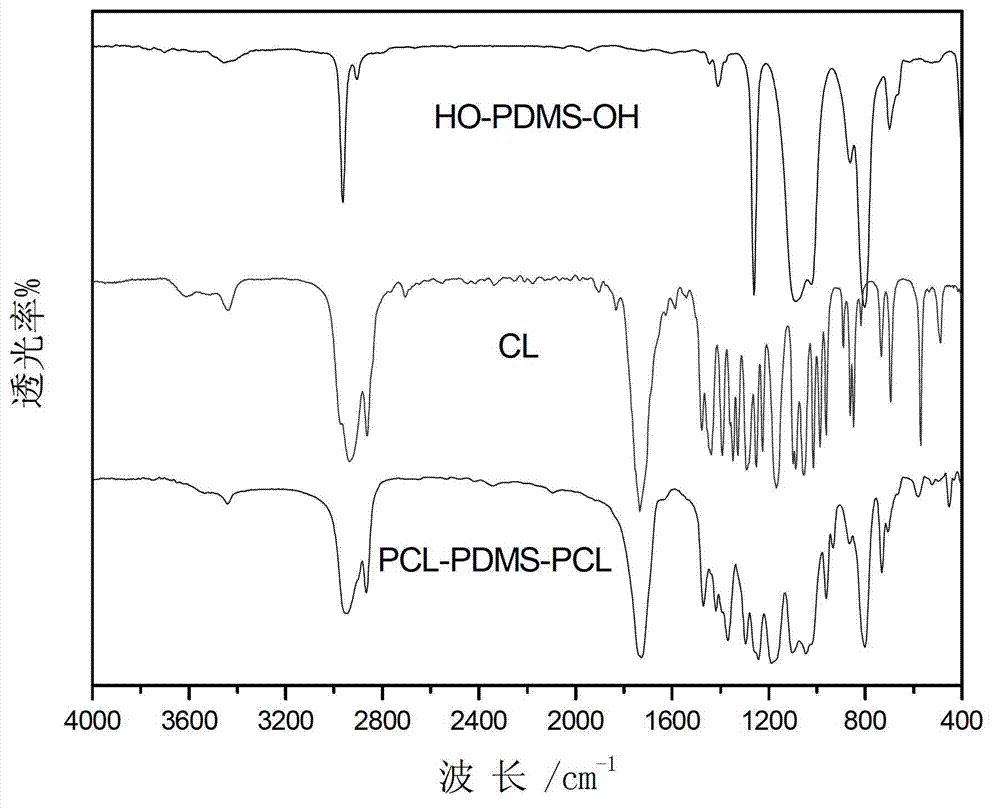
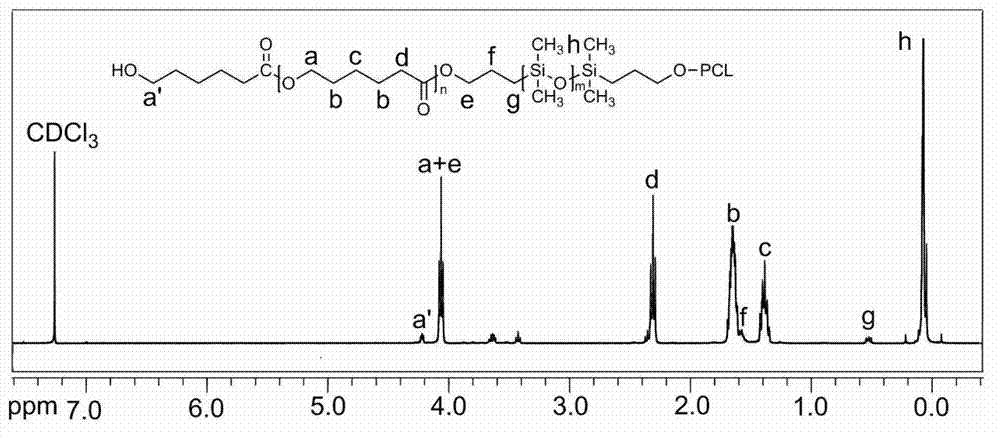
[0034] Example 1. Add 3g of hydroxypropyl silicone oil and 3g of caprolactone to a pre-dried 100mL three-neck flask, add 80mL of anhydrous toluene, connect the water separator, and azeotropically remove water at 125°C under the protection of argon. When 50mL is evaporated Add 40 mg of stannous octoate after the toluene, continue to distill 4 mL of toluene, replace the water separator as a reflux device, and lower the temperature to 120 ° C for 24 hours. After the reaction, cool down to 20°C and add 6mL of dichloromethane to dissolve the product, slowly add dropwise to 10 times the volume of liquid methanol at -5°C to obtain a white solid product, add 6mL of dichloromethane to dissolve the product again, and use -5°C Liquid methanol was precipitated twice to remove unreacted monomers, and the product was dried in a vacuum oven at 30°C to constant weight to obtain the stabilizer PCL-b-PDMS-b-PCL, the product quality was 5.3g, and the yield It was 88.3%, and its final molecular w...
example 2
[0035] Example 2. Add 4.5g of hydroxypropyl silicone oil and 3g of caprolactone to a pre-dried 100mL three-necked flask, add 70mL of anhydrous toluene, connect the water separator, and azeotropically remove water at 130°C under the protection of argon. Add 50 mg of stannous octoate after 45 mL of toluene, continue to distill 3.5 mL of toluene, replace the water separator as a reflux device, and cool down to 120 ° C for 24 hours. After the reaction, cool down to 25°C and add 8mL of dichloromethane to dissolve the product, slowly add dropwise to 10 times the volume of 0°C liquid methanol to obtain a white solid product, re-add 8mL of dichloromethane to dissolve the product, and again use 0°C liquid methanol Precipitate twice to remove unreacted monomers, place the product in a vacuum oven at 30°C and dry to constant weight to obtain the stabilizer PCL-b-PDMS-b-PCL, the product quality is 6.5g, and the yield is 87.3 %, the final molecular weight determined by NMR calculation is 1...
example 3
[0036] Example 3. Add 2.5g of hydroxypropyl silicone oil and 2.5g of caprolactone to a pre-dried 100mL three-neck flask, add 75mL of anhydrous toluene, connect the water separator, and azeotropically remove water at 120°C under the protection of argon. After removing 50 mL of toluene, add 30 mg of stannous octoate, continue to distill 4 mL of toluene, replace the water separator as a reflux device, and lower the temperature to 100°C for 24 hours. After the reaction, cool down to normal temperature and add 5mL of dichloromethane to dissolve the product, slowly drop into 10 times the volume of -18°C liquid methanol to obtain a white solid product, add 5mL of dichloromethane to dissolve the product, and again use -18°C liquid Precipitate with methanol twice to remove unreacted monomers, place the product in a vacuum drying oven at 30°C and dry to constant weight to obtain the stabilizer PCL-b-PDMS-b-PCL, the product quality is 4.7g, and the yield is 93.3%, the final molecular wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com