A kind of Ailamod slow-release multicomponent composition and preparation method thereof
A composition and multiple technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of multiple release factors, time lag, unstable osmotic pump and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
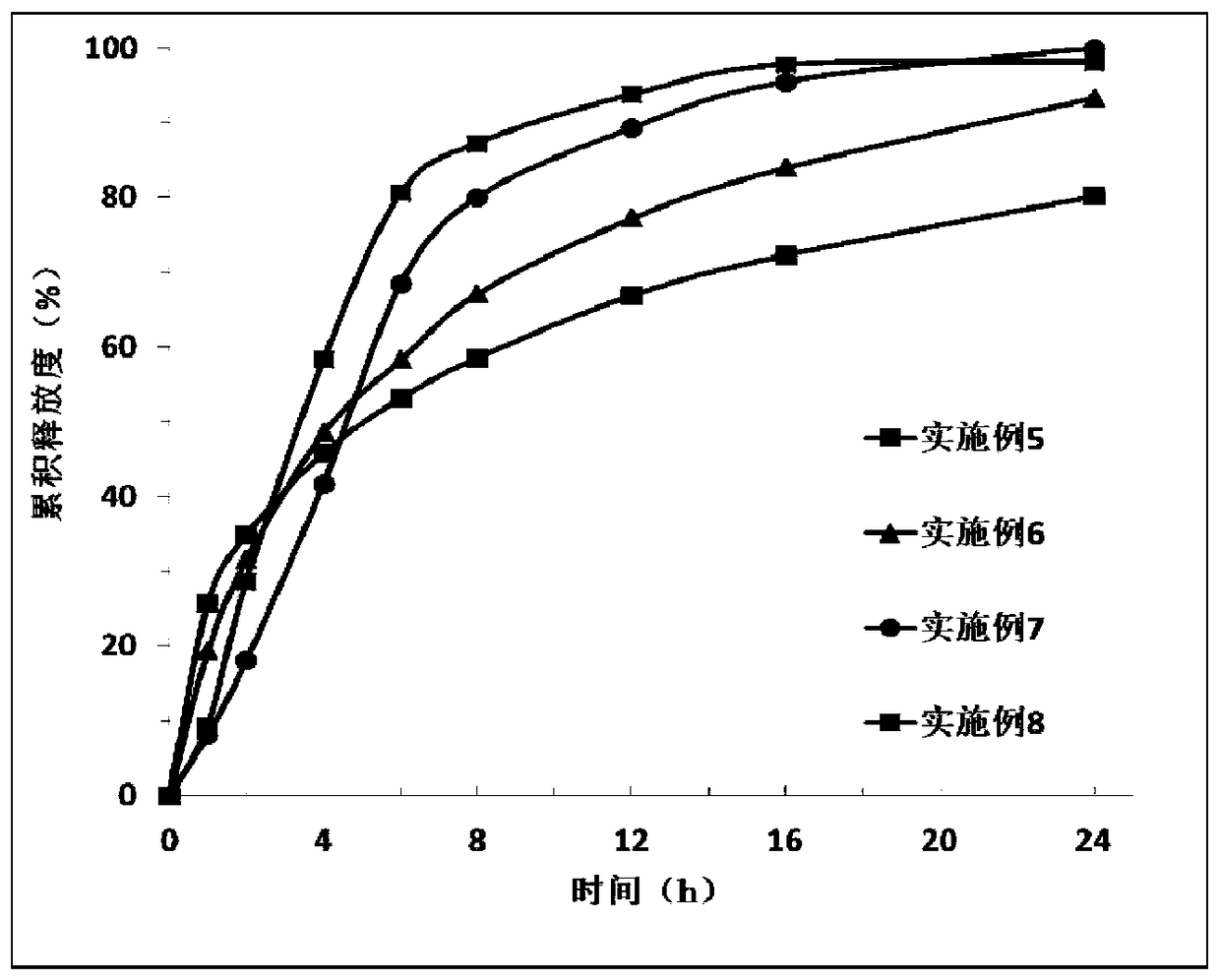
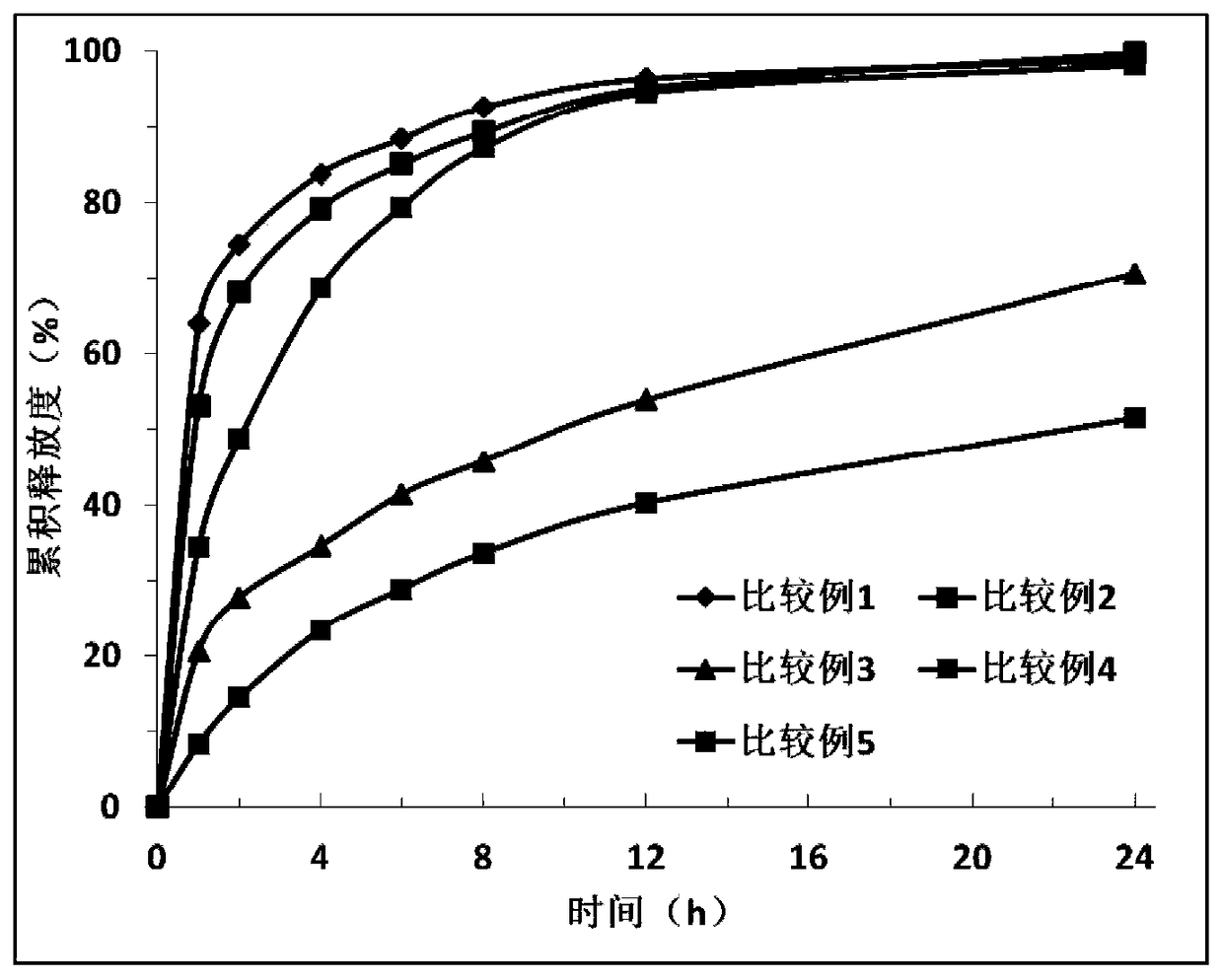
Examples
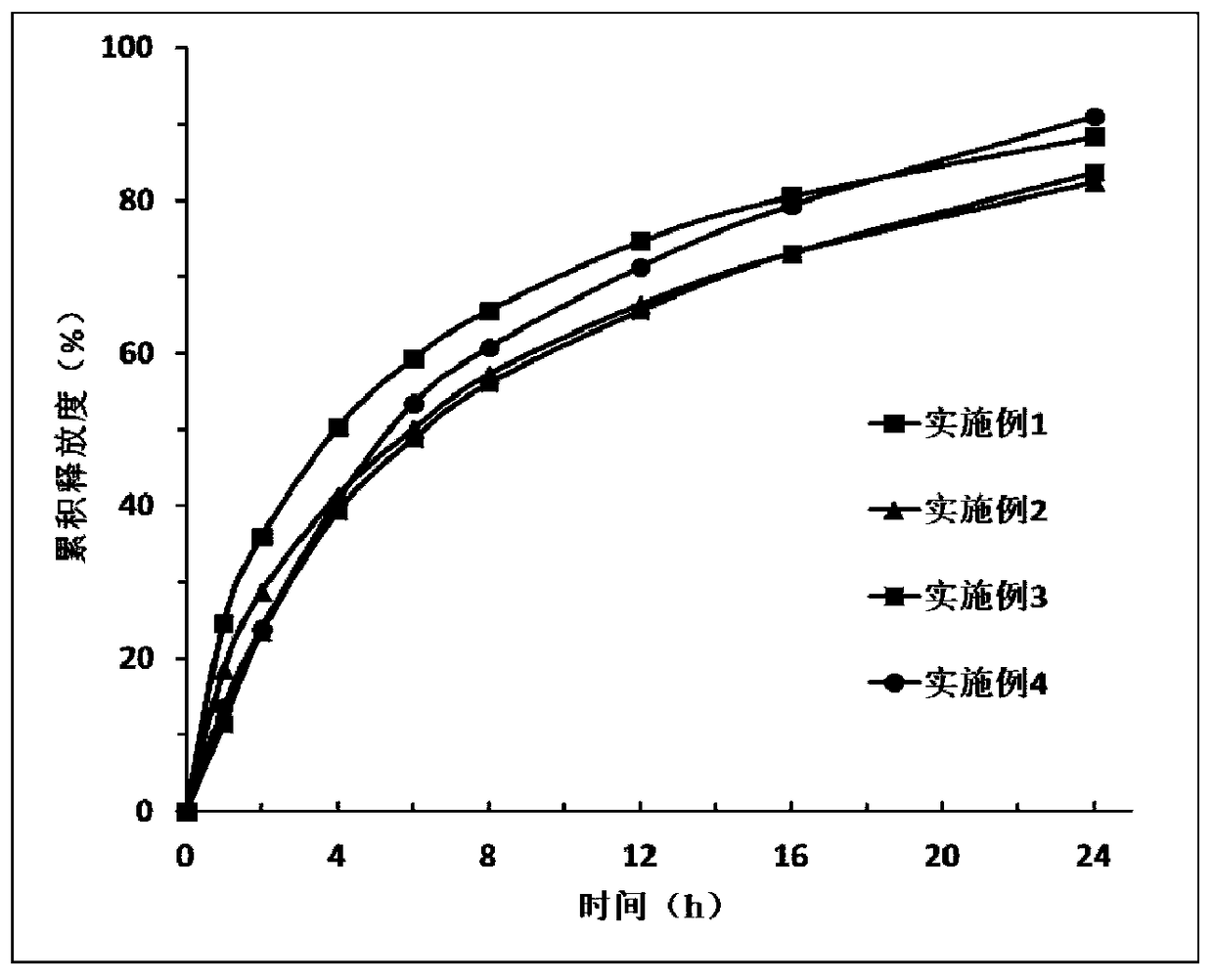
Embodiment 1
[0047] Use (spray method) (whether it is a conventional process, if not, it is necessary to explain in detail.) Spray 4.75g of plasticizer dibutyl sebacate into 9.5g of the skeleton carrier material ethyl cellulose 10 superior grade, to ethyl cellulose The base cellulose is plasticized, and then 33.25 g of a release modifier polyoxyethylene (PEO1000000) is added and mixed evenly to prepare a mixed carrier. A physical mixture was prepared by mixing 2.5 g of Iguratimod (source) with mixed carrier uniformly. Set the temperature of the hot-melt extruder (model, manufacturer) at 50°C. After the temperature rises to the set value and balances, the physical mixture is added to the hot-melt extruder, and the material is extruded from the die hole of the head; After cooling to room temperature, the mixture was pulverized and subjected to in vitro release experiments.
Embodiment 2
[0049] Mix 40.5 g of the skeleton carrier material ethylene / vinyl acetate copolymer with 2.25 g of the release modifier xanthan gum and 2.25 g of carbomer 974P to prepare a mixed carrier. Mix Iguratimod 5g with mixed carrier uniformly to prepare physical mixture. Set the temperature of the hot-melt extruder at 70°C. After the temperature rises to the set value and balances, add the physical mixture to the hot-melt extruder, and the material is extruded from the die hole of the head; after cooling the extrudate to room temperature Crush and perform in vitro release experiments.
Embodiment 3
[0051] Mix 21.6 g of the skeleton carrier material ethyl cellulose 10 superior grade with 14.4 g of the plasticizer polyethylene glycol (PEG400) and 4 g of the release modifier carbomer 974P to prepare a mixed carrier. Mix Iguratimod 10g with mixed carrier uniformly to prepare physical mixture. Set the temperature of the hot-melt extruder at 90°C. After the temperature rises to the set value and balances, add the physical mixture into the hot-melt extruder, and the material is extruded from the die hole of the head; after cooling the extrudate to room temperature Crush and perform in vitro release experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com