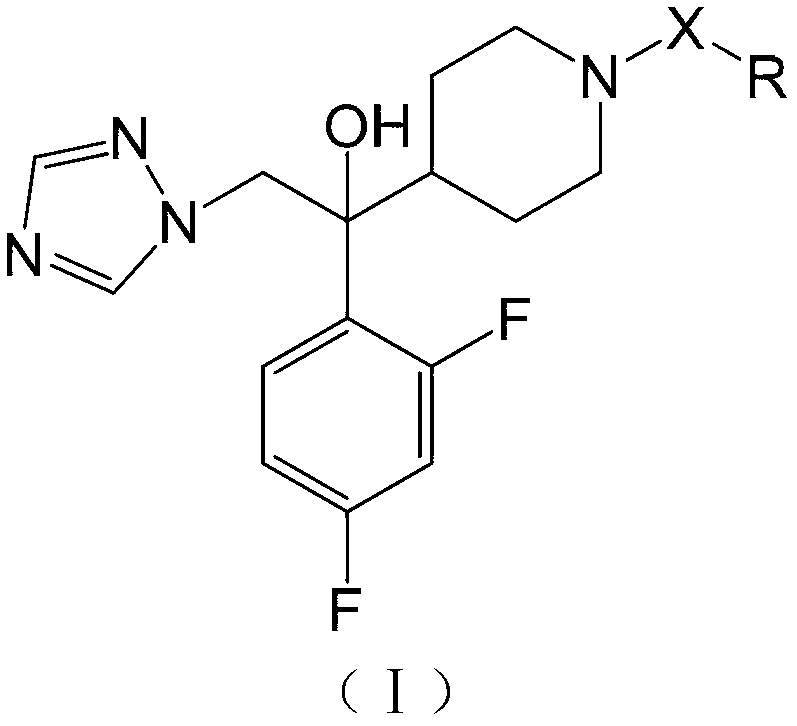
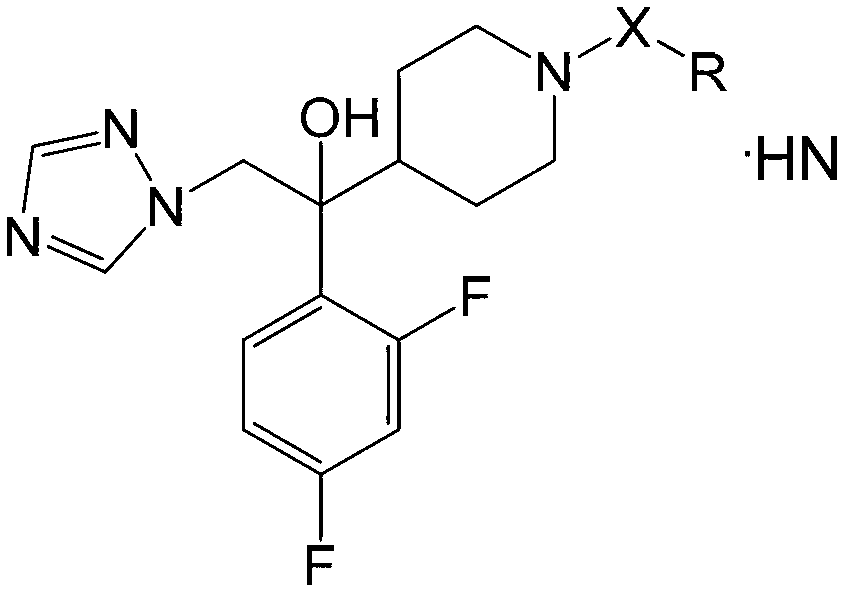
Azole antifungal compound, its preparation method and application
A technology of a compound and a target compound, applied in the field of azole antifungal compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
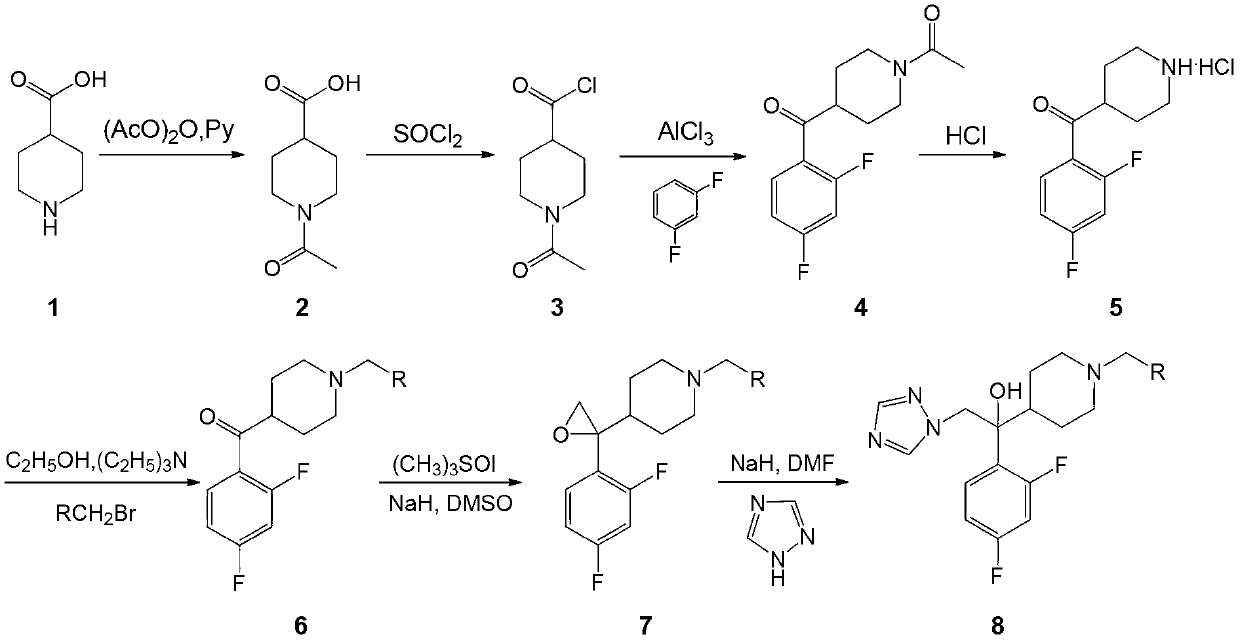
Embodiment 1
[0078] Embodiment 1: Preparation of 1-ethoxyformyl-4-piperidinecarboxylic acid (2)
[0079] Add 4-piperidinecarboxylic acid (1, 10.0g, 77.4mmol), acetic anhydride (25mL, 264.5mmol), pyridine (6.3mL, 77.7mmol) into the reaction flask, and heat to reflux for 4h. Evaporate excess acetic anhydride under reduced pressure, cool to room temperature, add a mixed solvent of ethanol and ether (30mL, V / V=2:3), stir and crystallize, filter with suction, and dry to obtain white solid 1-ethoxyformyl-4 - 10.1 g of piperidinecarboxylic acid (2), yield 76%.
Embodiment 2
[0080] Embodiment 2: Preparation of 1-ethoxyformyl-4-piperidinecarbonyl chloride (3)
[0081] Add 1-ethoxyformyl-4-piperidinecarboxylic acid (2, 10.1g, 50.5mmol) and thionyl chloride (10.7mL, 201.6mmol) into an eggplant-shaped flask, stir and heat to 65°C for 4h. Thionyl chloride was distilled off under reduced pressure to obtain 10.9 g of yellow oily substance 1-ethoxyformyl-4-piperidinecarbonyl chloride (3), with a yield of 98%.
Embodiment 3
[0082] Example 3: Preparation of 2,4-difluorophenyl-(1-ethoxyformyl-4-piperidinyl)methanone (4)
[0083] Anhydrous aluminum trichloride (10.6g, 80mmol) and m-difluorobenzene (6mL, 60mol) were placed in a 100mL three-neck flask, stirred at room temperature, and slowly dropped into 1-carboethoxy-4-piperidinemethyl Acyl chloride (3, 10.9g, 19.6mmol), continue to stir at room temperature for 30 minutes after the dropwise addition, slowly raise the temperature, heat to reflux for 3h, cool to room temperature, evaporate m-difluorobenzene under reduced pressure, and pour the reaction solution as usual into 50 mL of ice water, extracted with dichloromethane (80 mL×3), combined with dichloromethane, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, evaporated under reduced pressure to remove dichloromethane, and dried to obtain light yellow oil 2 , 10.7 g of 4-difluorophenyl-(1-ethoxyformyl-4-piperidinyl)methanone (4), yield 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com