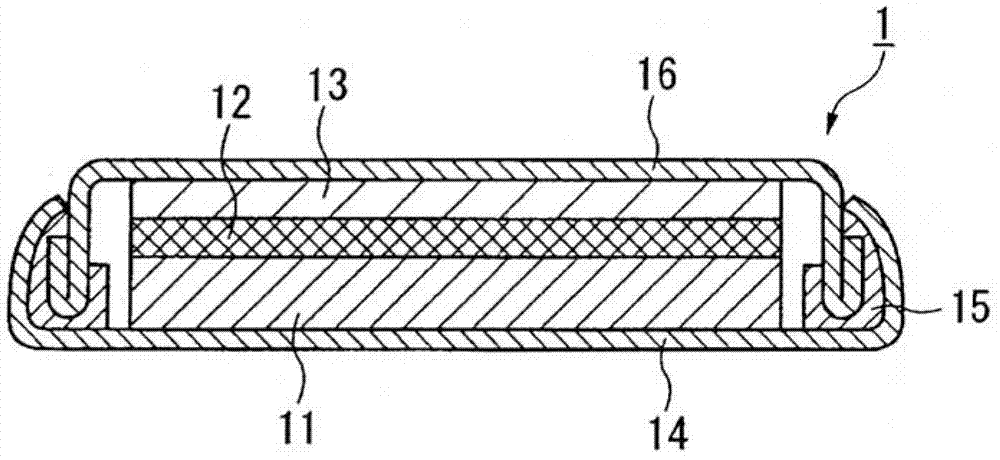
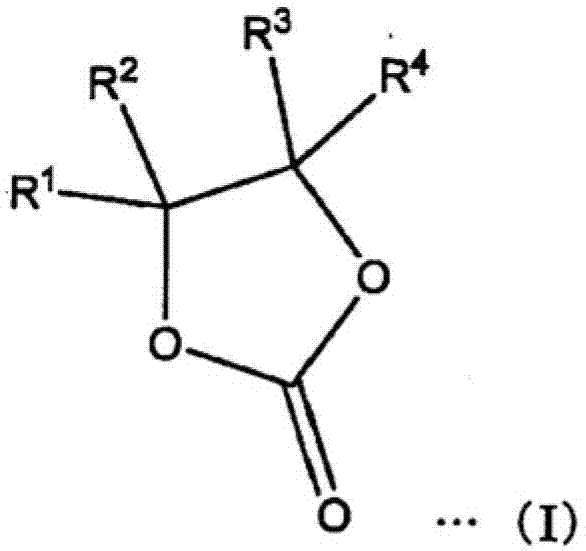
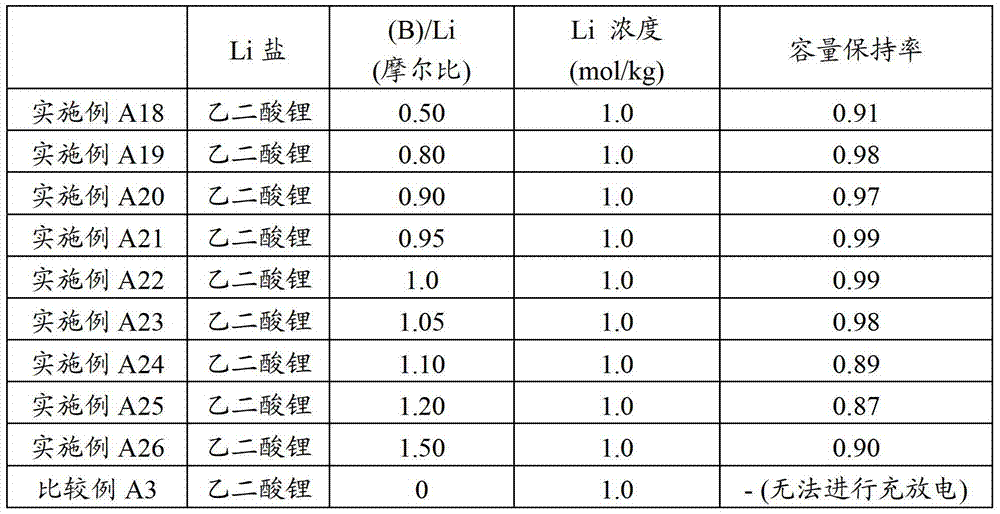
Electrolyte, electrolytic solution, gel electrolyte, electrolyte membrane, manufacturing method of gel electrolyte battery, and lithium ion secondary battery
A gel electrolyte and electrolyte technology, applied in the field of electrolytes, can solve the problems of thermal instability, high cost, easy hydrolysis, etc., and achieve the effects of full charge-discharge characteristics and excellent cycle life characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0150] Hereinafter, the present invention will be described in further detail through specific examples. However, the present invention is not limited at all by the Examples shown below.
[0151] (1) Chemical substances used
[0152] The chemical substances used in this example are shown below.
[0153] ・(A) Raw material of lithium salt of organic acid
[0154] Formic acid (manufactured by Aldrich)
[0155] Acetic acid (manufactured by Aldrich)
[0156] Propionic acid (manufactured by Aldrich)
[0157] Butyric acid (manufactured by Aldrich)
[0158] Isobutyric acid (manufactured by Aldrich)
[0159] Oxalic acid (manufactured by Wako Pure Chemical Industries, Ltd.)
[0160] Lithium oxalate (manufactured by Aldrich)
[0161] Succinic acid (manufactured by Aldrich)
[0162] Adipic acid (manufactured by Aldrich)
[0163] Lithium hydroxide monohydrate (LiOH·H 2 O) (manufactured by Aldrich Corporation)
[0164] ·(B) Boron compounds
[0165] Boron trifluoride diethyl ether...
Embodiment A1
[0204]
[0205] Measure the lithium acetate (0.182g) that obtains in above-mentioned (2-2) in sample bottle, BF 3 O(C 2 h 5 ) 2 (0.392g), a mixed solvent (EC:DMC=30:70 (volume ratio)) of EC and DMC as an organic solvent, mix and make the concentration of the lithium atom in lithium acetate be 1.0mol / kg, obtain thus electrolyte.
[0206]
[0207] A negative electrode (manufactured by Hosen Co., Ltd.) and a positive electrode (manufactured by Hosen Co., Ltd.) were punched out into a disc shape with a diameter of 16 mm. In addition, glass fibers were punched out into a disc shape with a diameter of 17 mm as a separator.
[0208] The obtained positive electrode, separator, and negative electrode were sequentially stacked in a SUS battery container (CR2032), and the electrolyte solution obtained above was impregnated into the separator, negative electrode, and positive electrode, and a SUS plate (thickness 1.2 mm, 16 mm in diameter) and capped to manufacture a coin-type ba...
Embodiment A2
[0210]
[0211] Measure lithium succinate (0.377g), BF obtained in the above (2-6) in the sample bottle 3 O(C 2 h 5 ) 2 (0.863g), the mixed solvent (EC:DMC=30:70 (volume ratio)) of EC and DMC as organic solvent, mix and make the concentration of the lithium atom in lithium succinate be 1.0mol / kg, by This yields an electrolyte solution.
[0212]
[0213] A coin-type battery was manufactured in the same manner as in Example A1 except that the electrolytic solution obtained in this example was used instead of the electrolytic solution obtained in Example A1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com