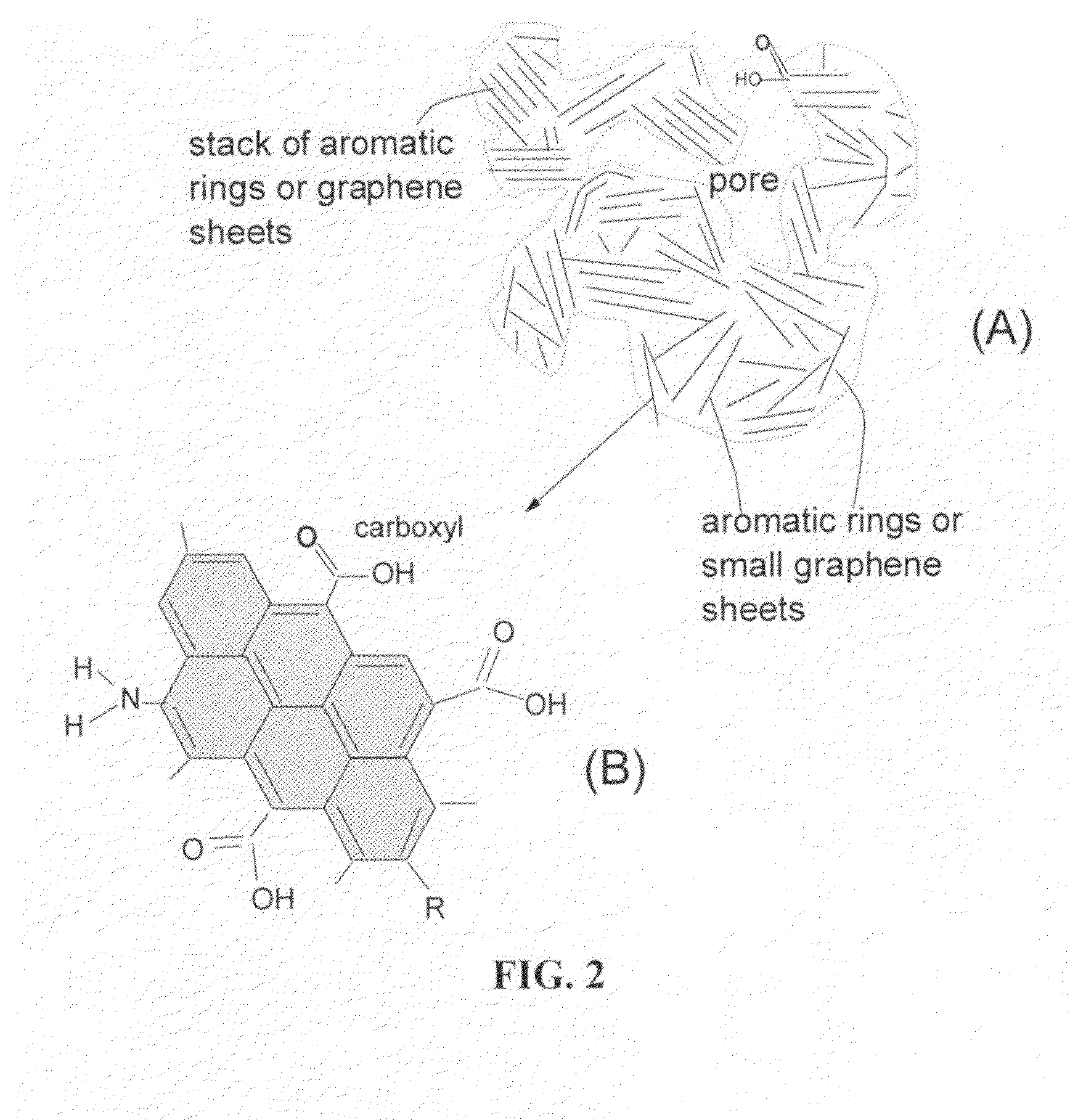
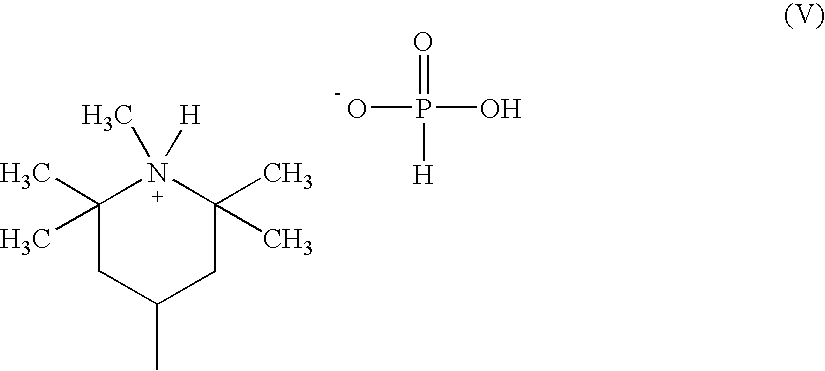
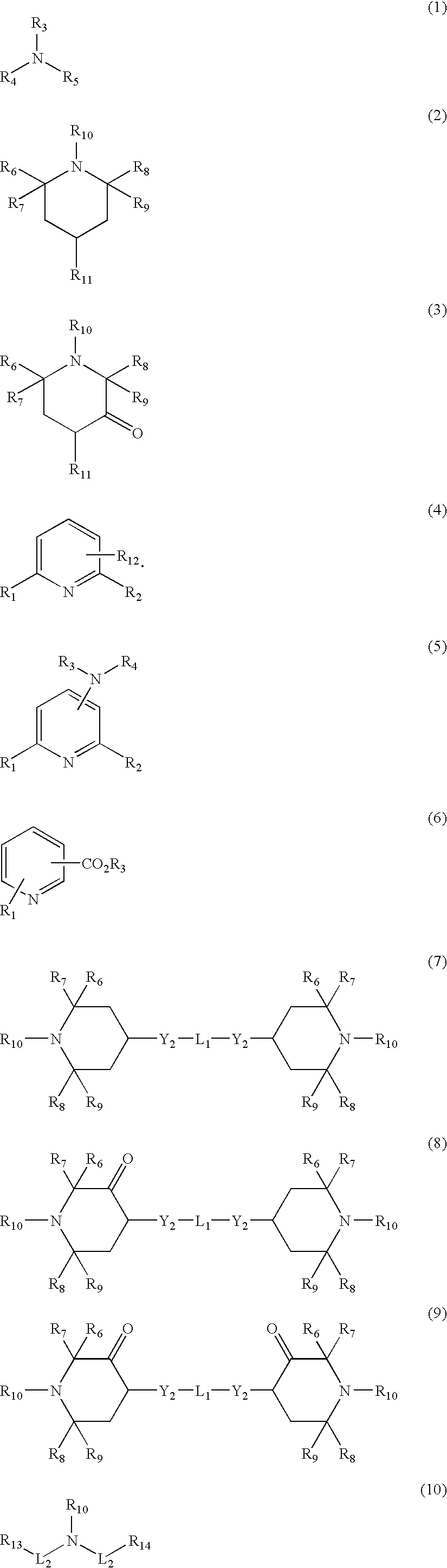
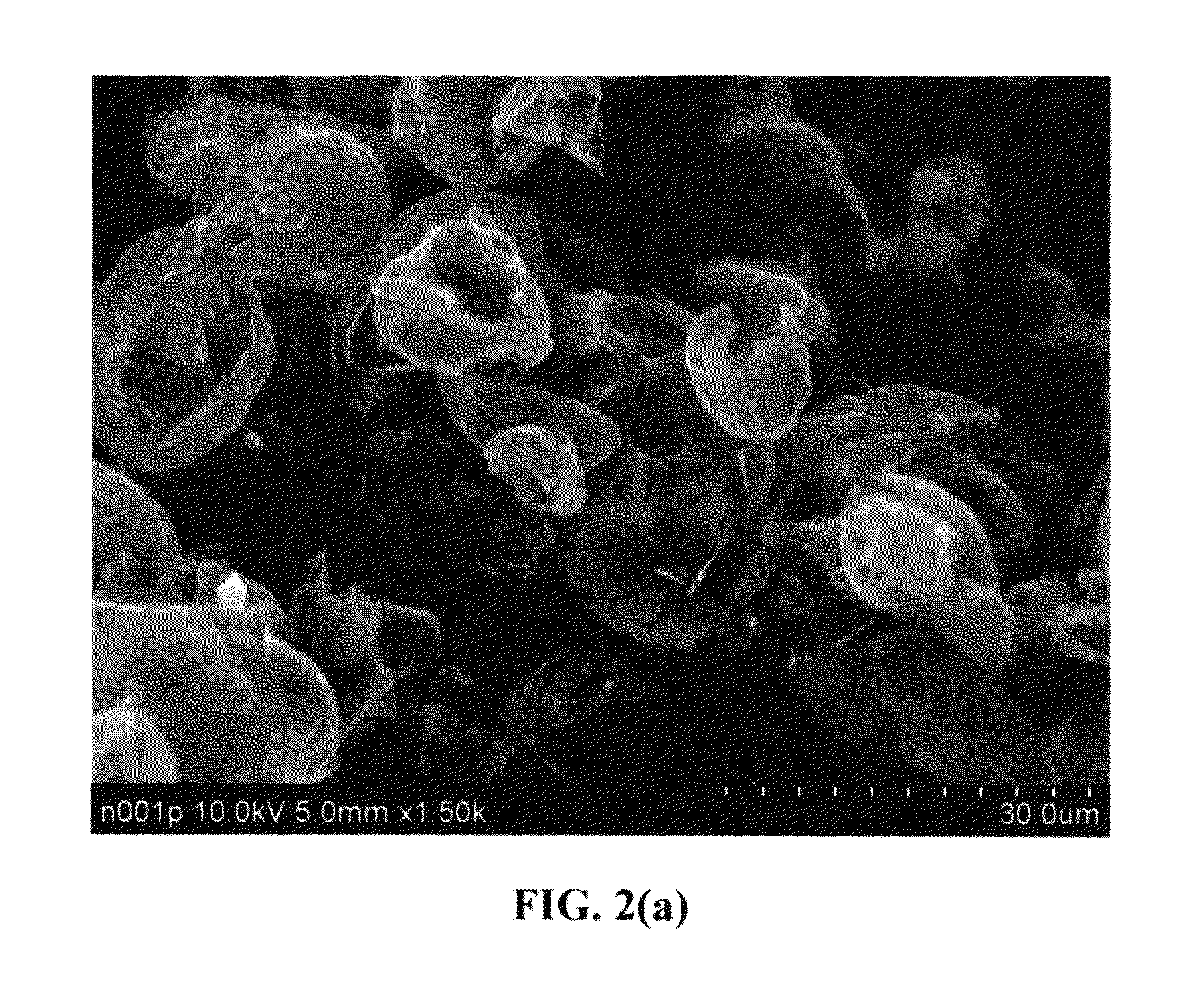
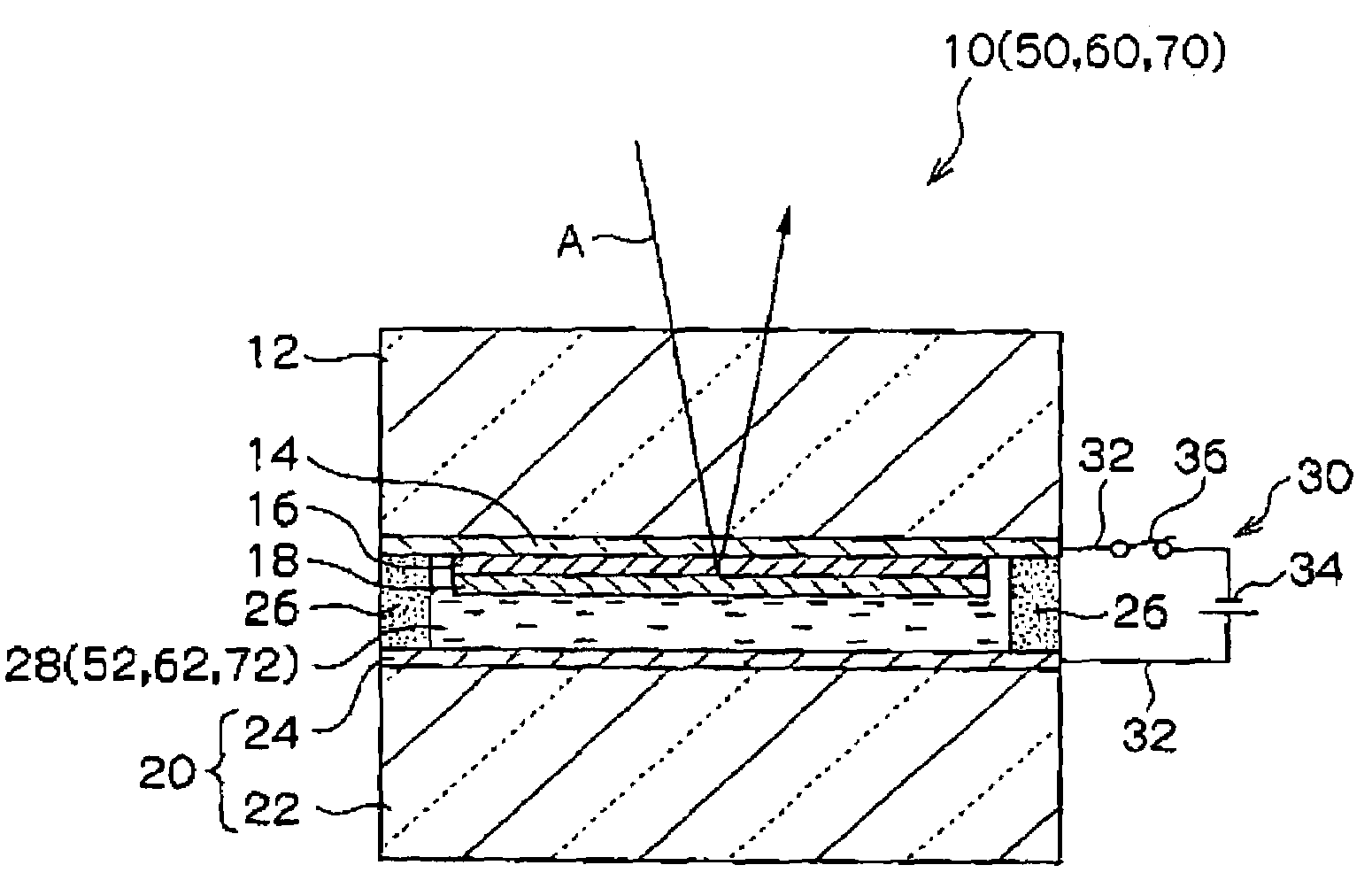
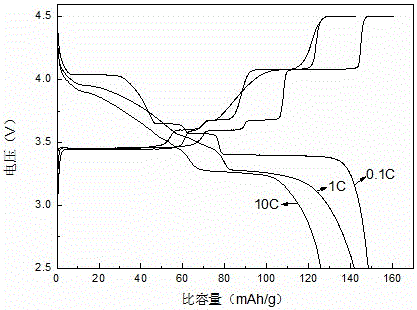
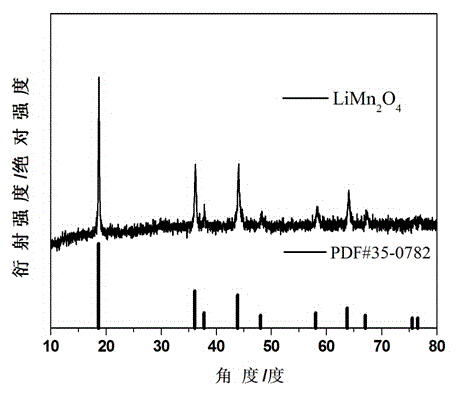
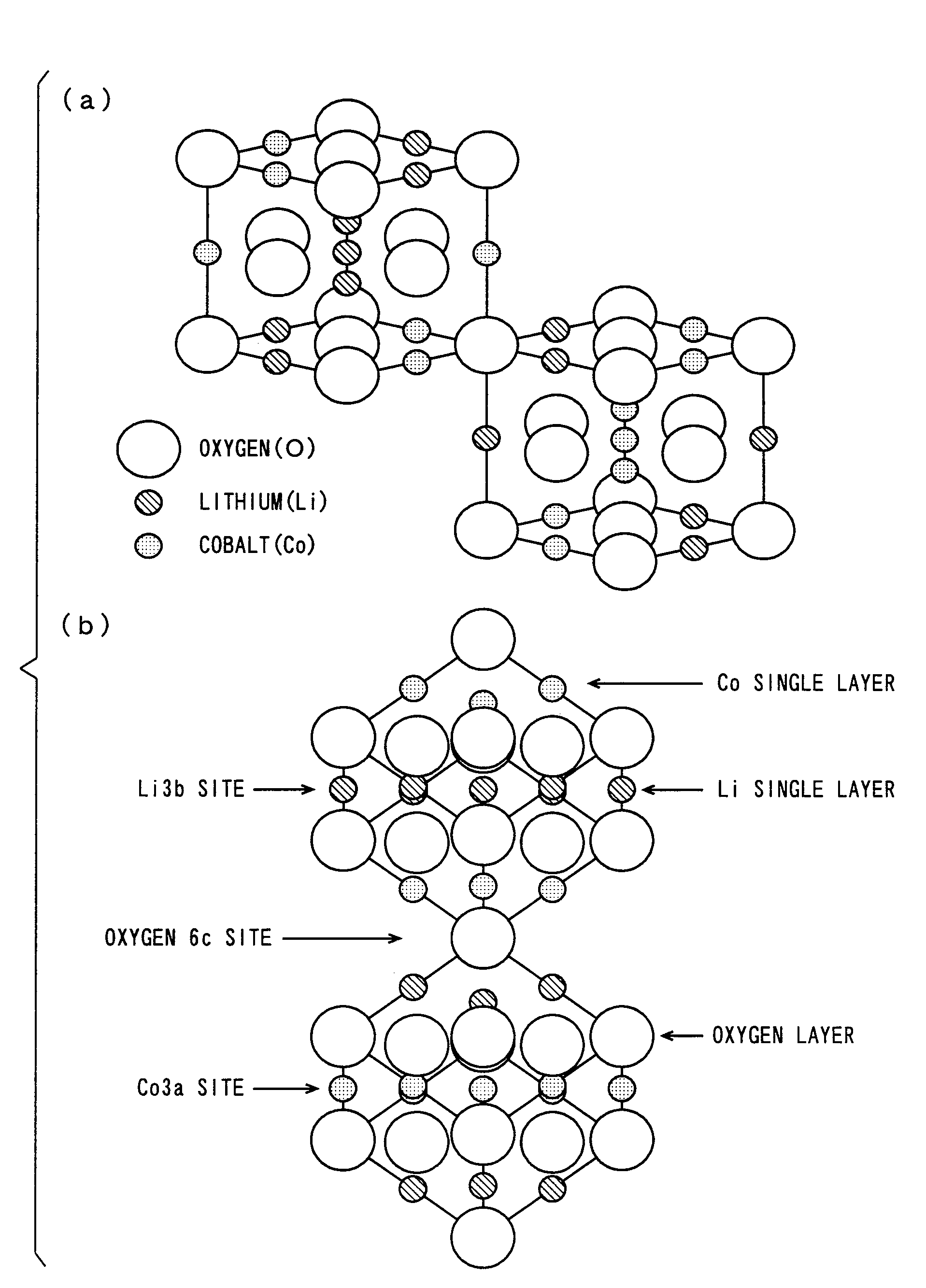
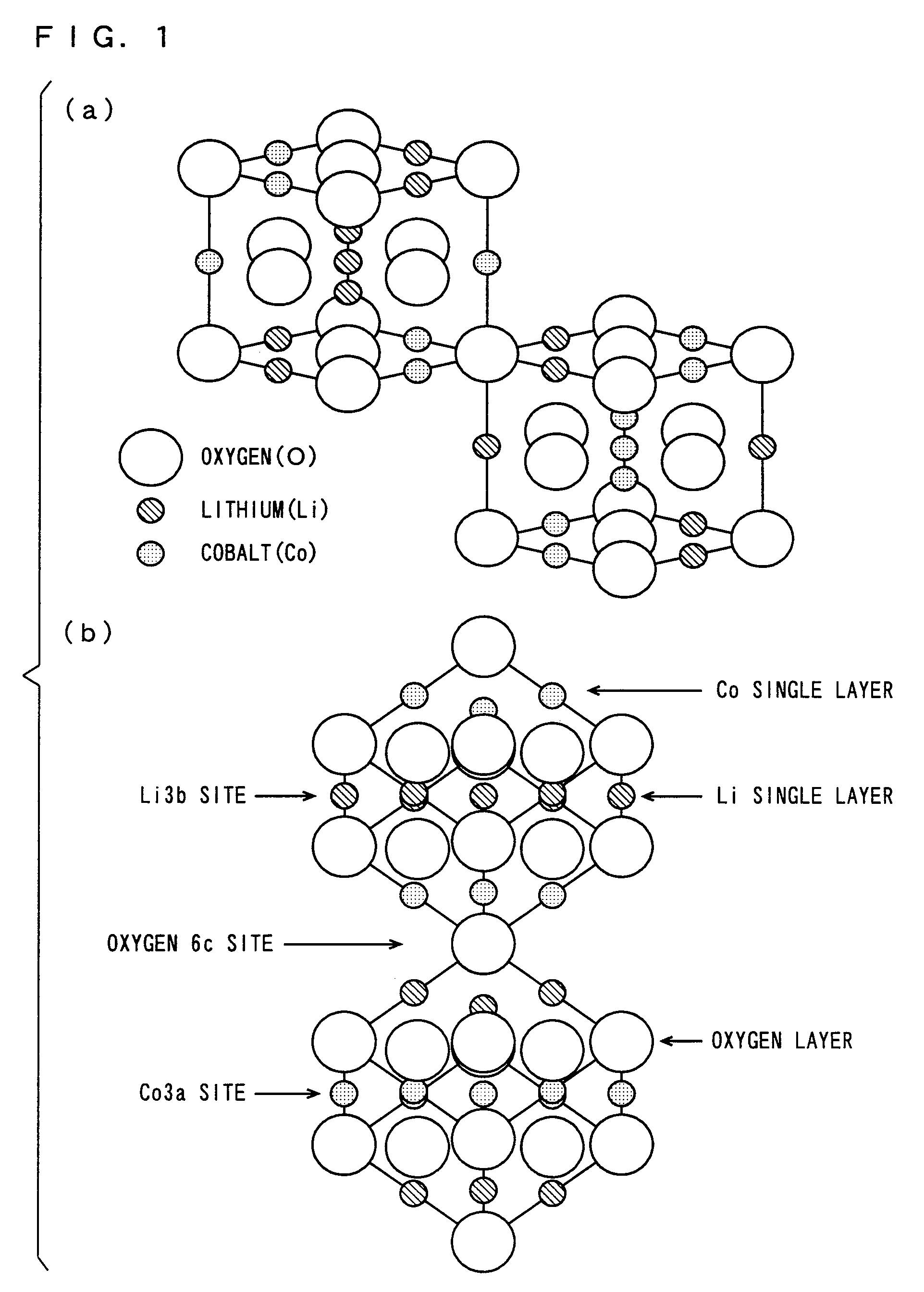
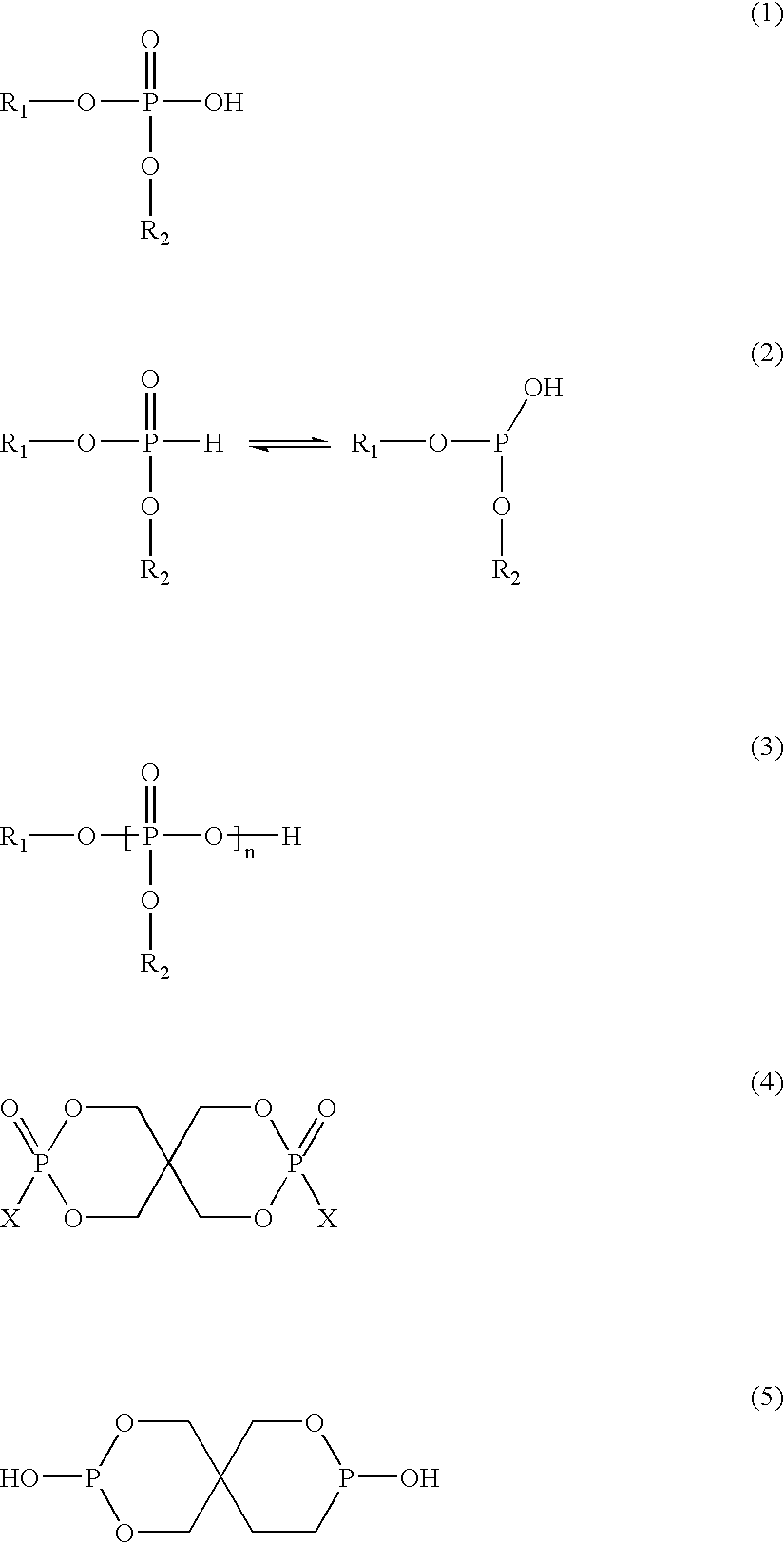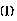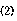Patents
Literature
97 results about "Lithium atom" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A lithium atom is an atom of the chemical element lithium. Lithium is composed of three electrons bound by the electromagnetic force to a nucleus containing three protons along with either three or four neutrons, depending on the isotope, held together by the strong force. Similarly to the case of the helium atom, a closed-form solution to the Schrödinger equation for the lithium atom has not been found. However, various approximations, such as the Hartree–Fock method, can be used to estimate the ground state energy and wavefunction of the atom. The quantum defect is a value that describes the deviation from hydrogenic energy levels.
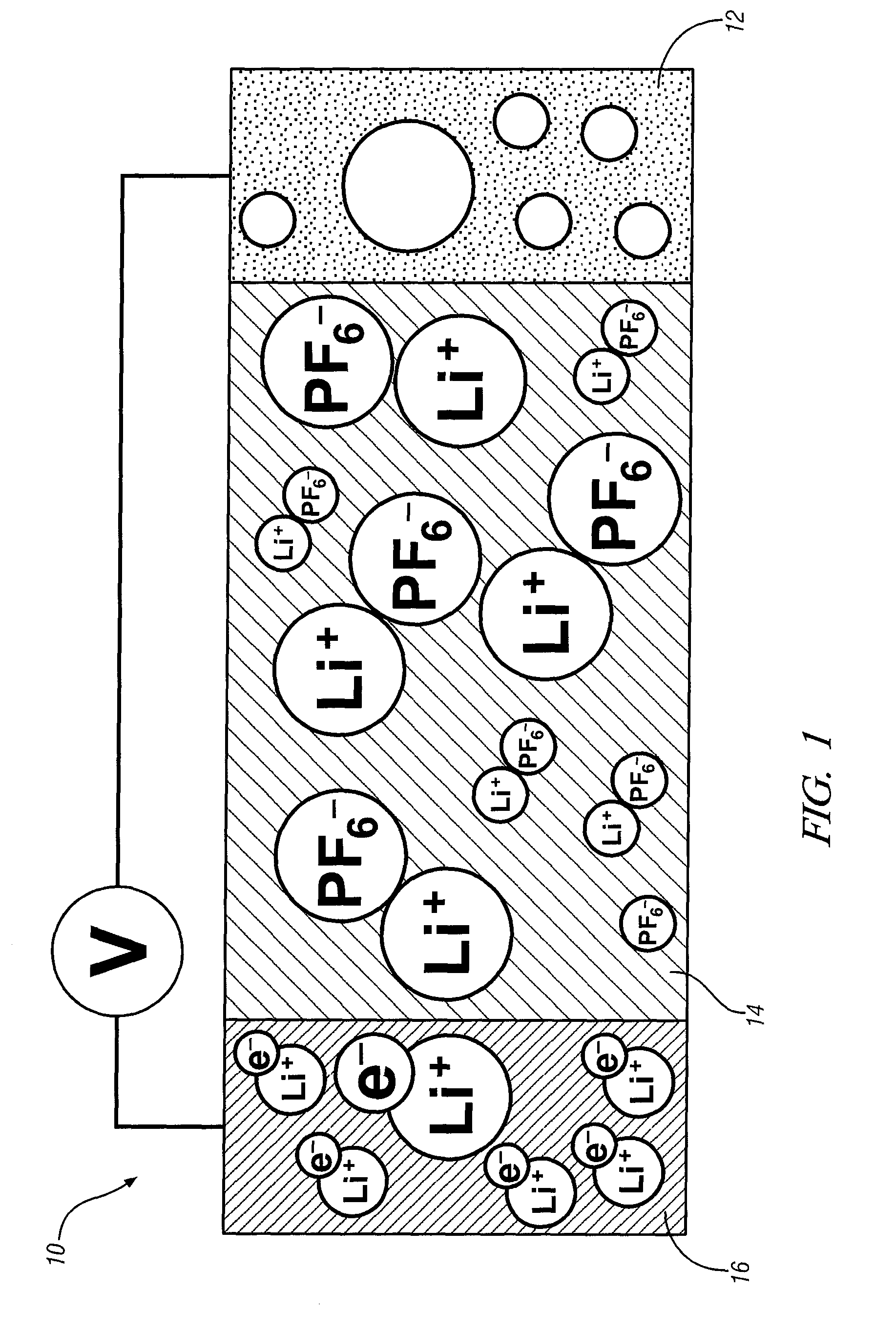
Non-aqueous electrolytes for lithium electrochemical cells
InactiveUS6852446B2Improve conductivityImprove stabilityAlkaline accumulatorsOrganic electrolyte cellsHydrogen atomHydrogen
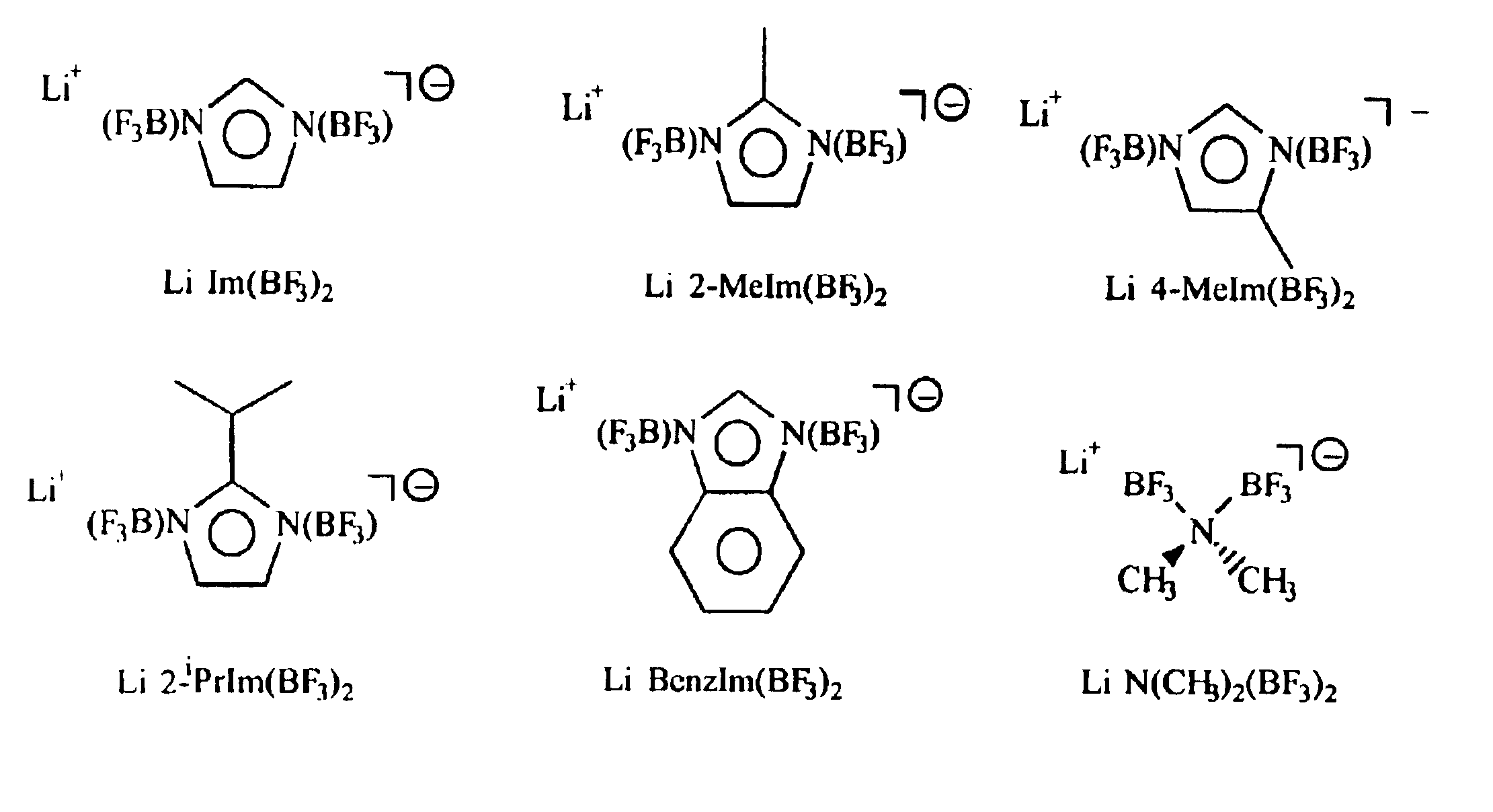
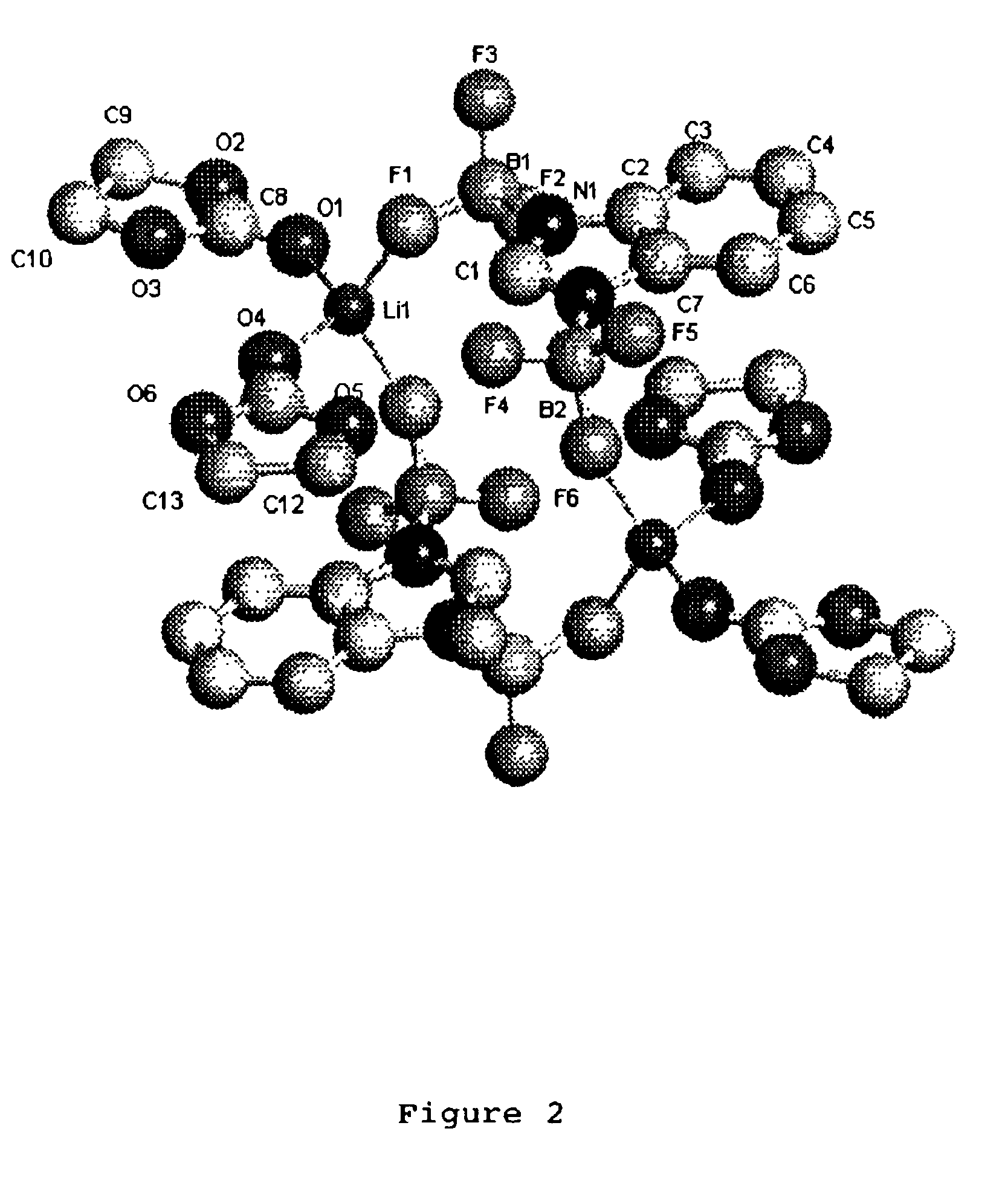
A non-aqueous electric current producing electrochemical cell is provided comprising an anode and a cathode, an ionically permeable separator interposed between the anode and the cathode, and a non-aqueous electrolyte, the electrolyte comprising an ionically conducting salt in a non-aqueous medium, the ionically conducting salt corresponding to the formula:M+(Z*(J*)j(X*)x)−, wherein:M is a lithium atom,Z* is an anion group containing two or more Lewis basic sites and comprising less than 50 atoms not including hydrogen atoms,J* independently each occurance is a Lewis acid coordinated to at least one Lewis basic site of Z*, and optionally two or more such J* groups may be joined together in a moiety having multiple Lewis acidic functionality,X* independently each occurrence is selected from the group consisting of H, C1-C4 alkyl, alkoxide, halide and mixtures thereof,j is an integer from 2 to 12, andx is an integer from 0 to 4.
Owner:EAGLE PICHER TECH LLC
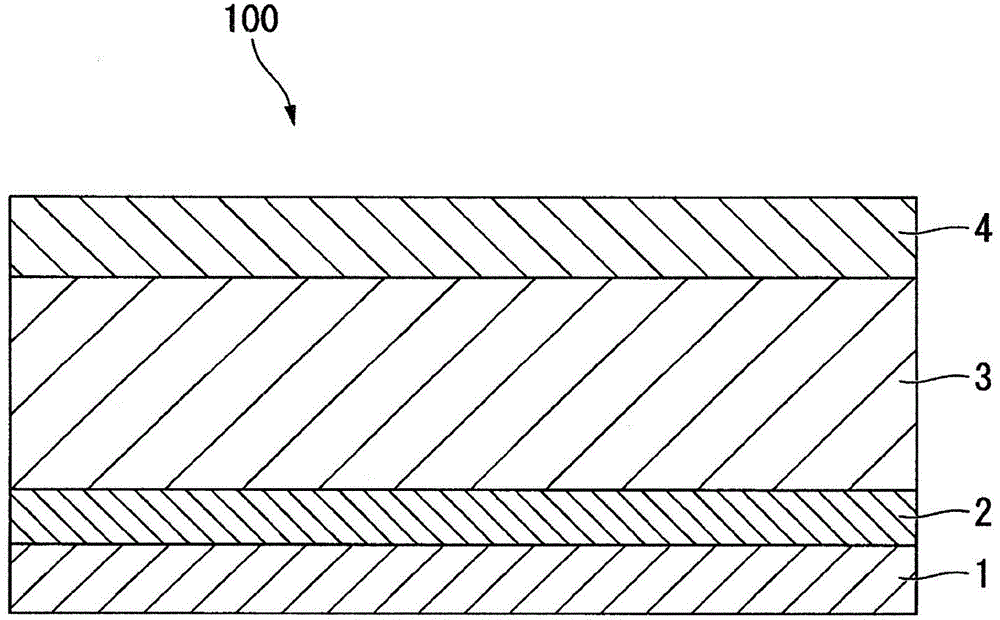
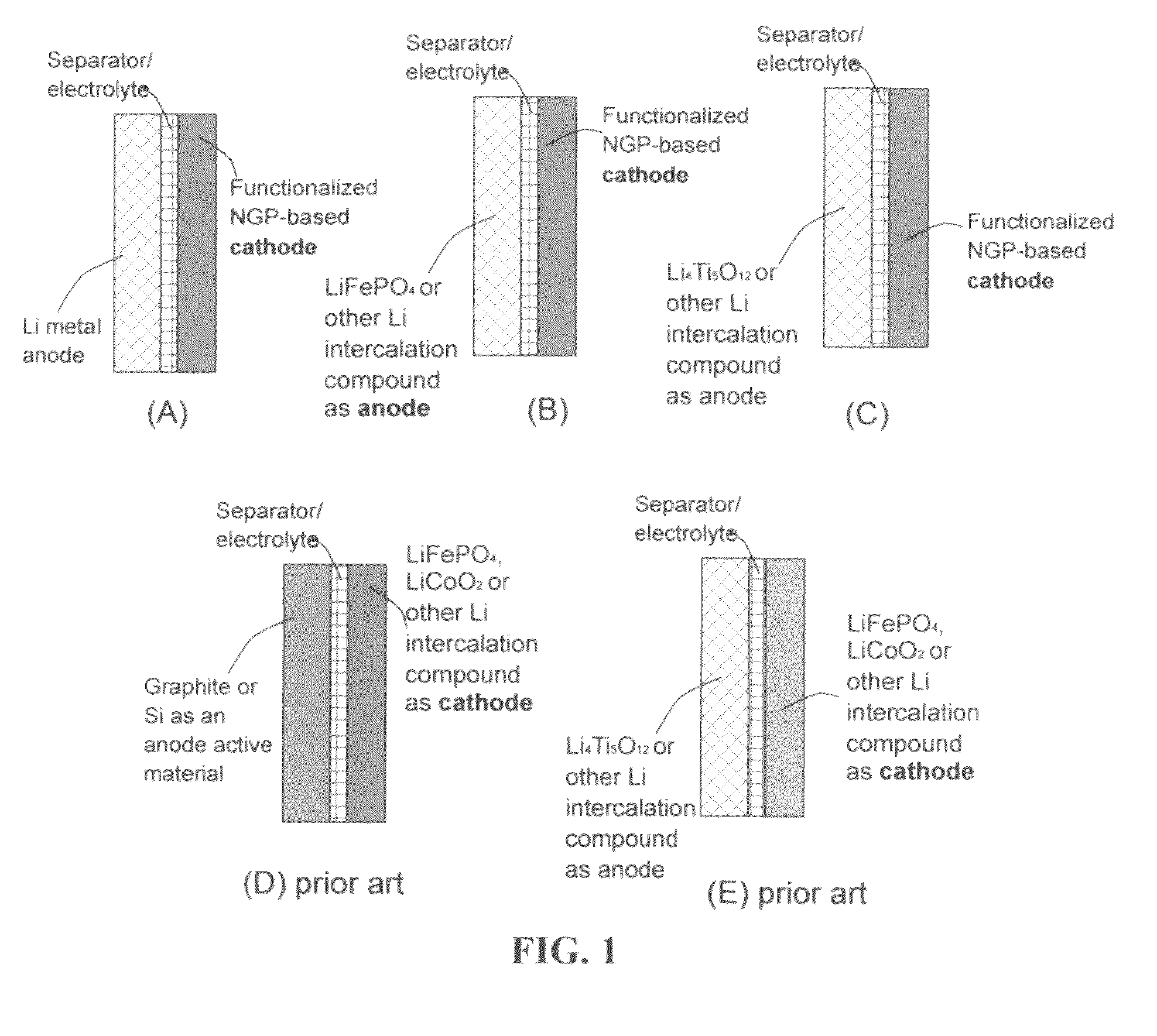
Lithium super-battery with a functionalized nano graphene cathode
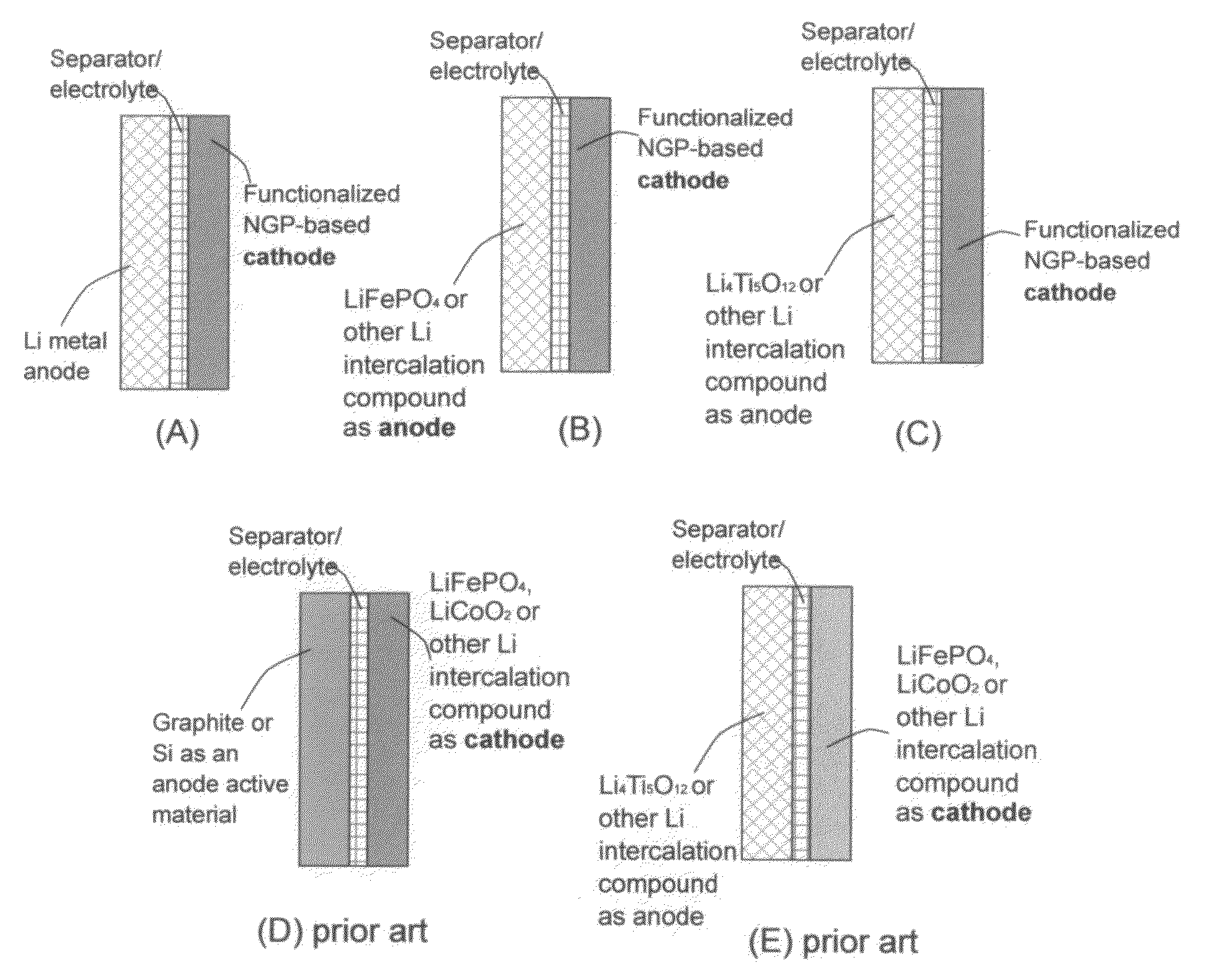
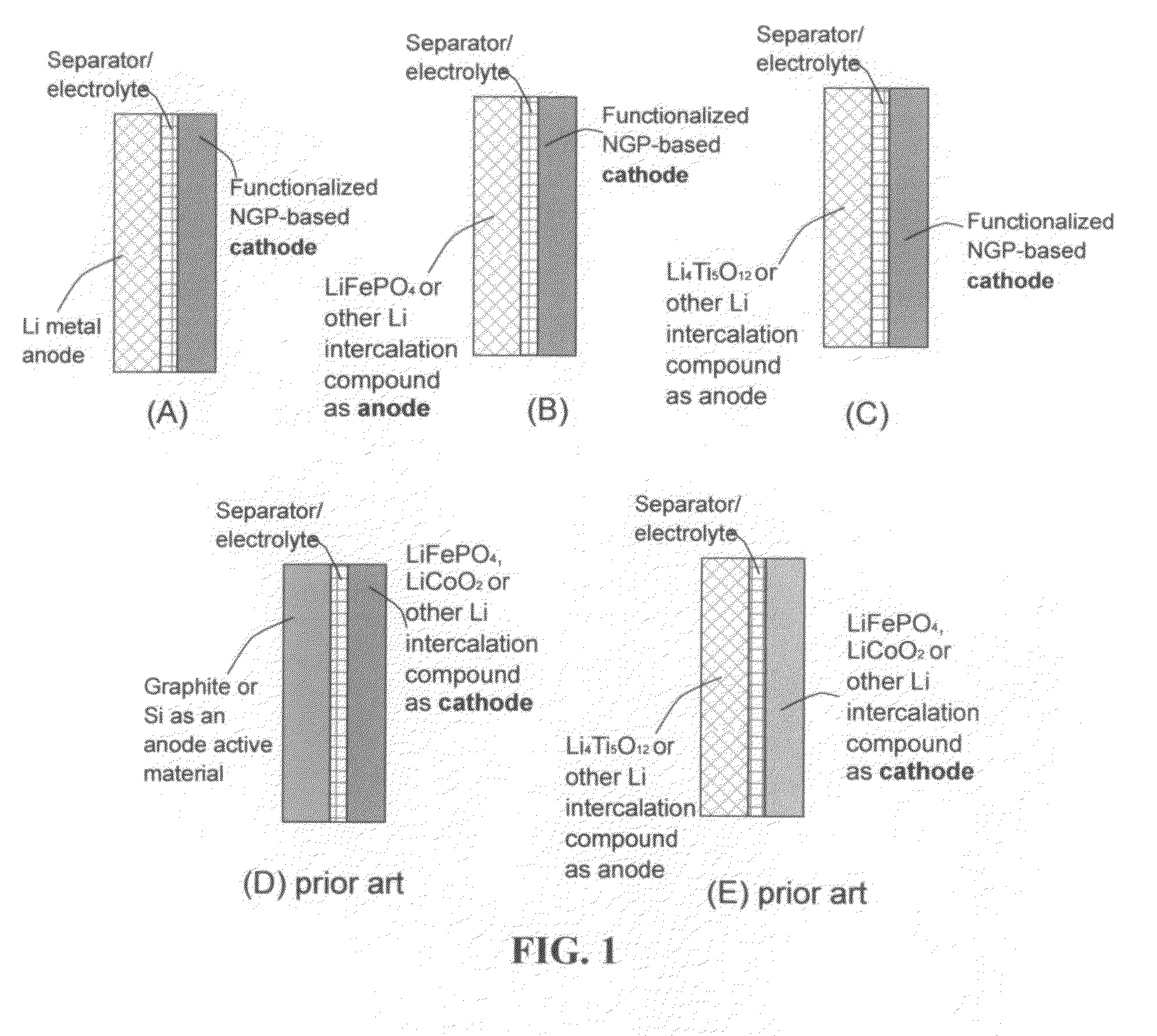
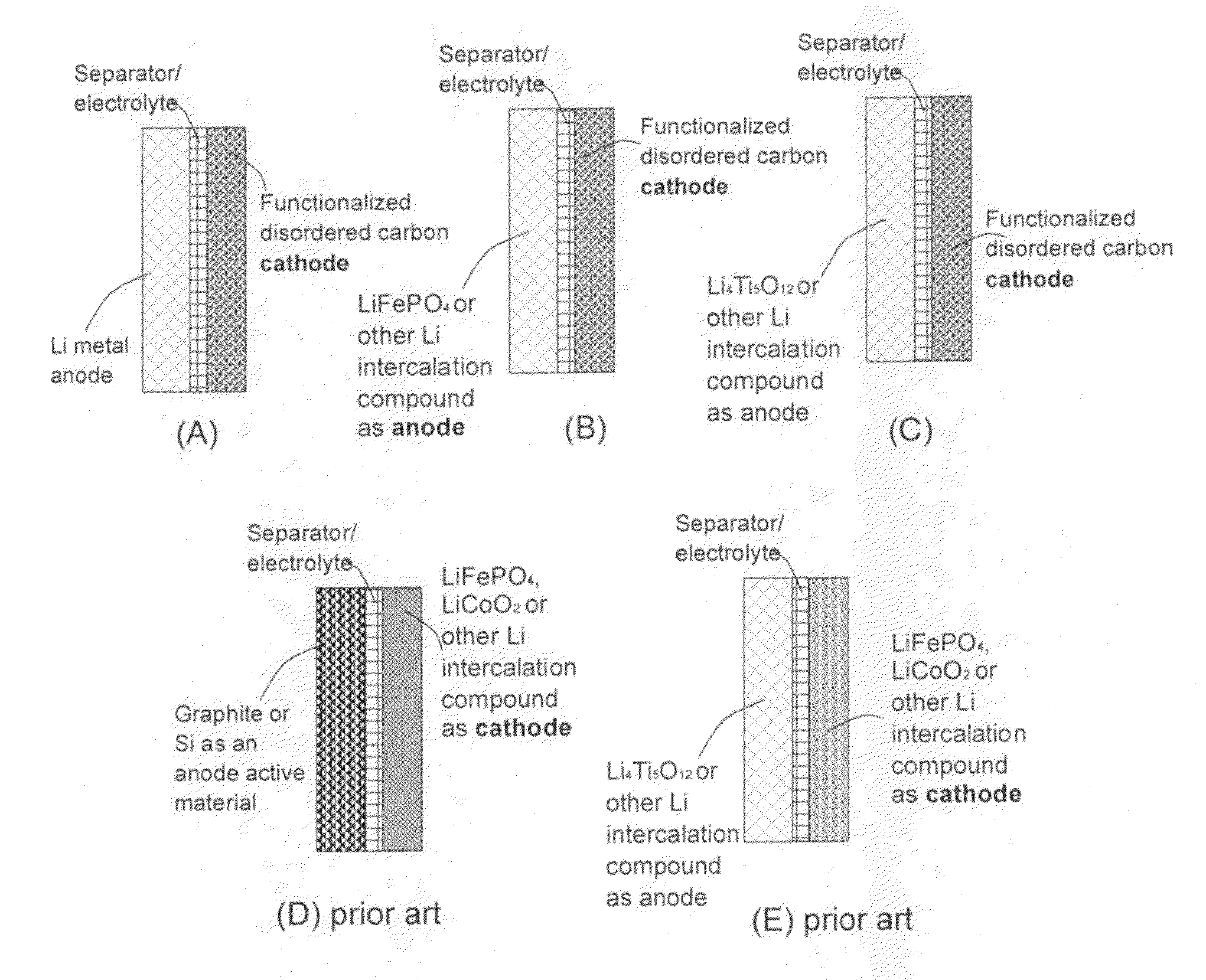
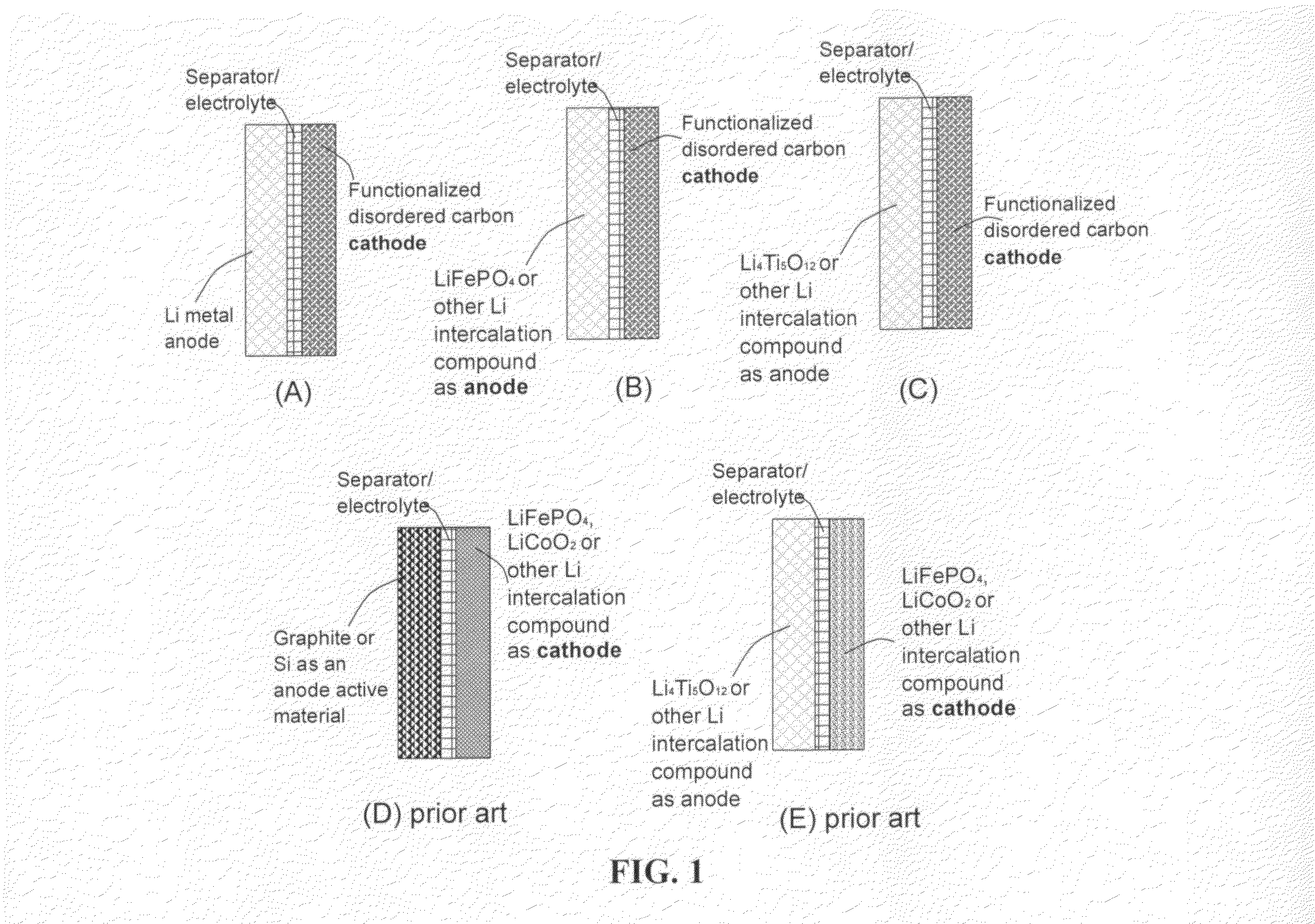
An electrochemical energy storage device, lithium super-battery, comprising a positive electrode, a negative electrode, a porous separator disposed between the two electrodes, and a lithium-containing electrolyte in physical contact with the two electrodes, wherein the positive electrode comprises a plurality of chemically functionalized nano graphene platelets (f-NGP) or exfoliated graphite having a functional group that reversibly reacts with a lithium atom or ion. In a preferred embodiment, a lithium super-battery having a f-NGP positive electrode and Li4Ti5O12 negative electrode exhibits a gravimetric energy ˜5 times higher than conventional supercapacitors and a power density ˜10 times higher than conventional lithium-ion batteries. This device has the best properties of both the lithium ion battery and the supercapacitor.
Owner:GLOBAL GRAPHENE GRP INC +1
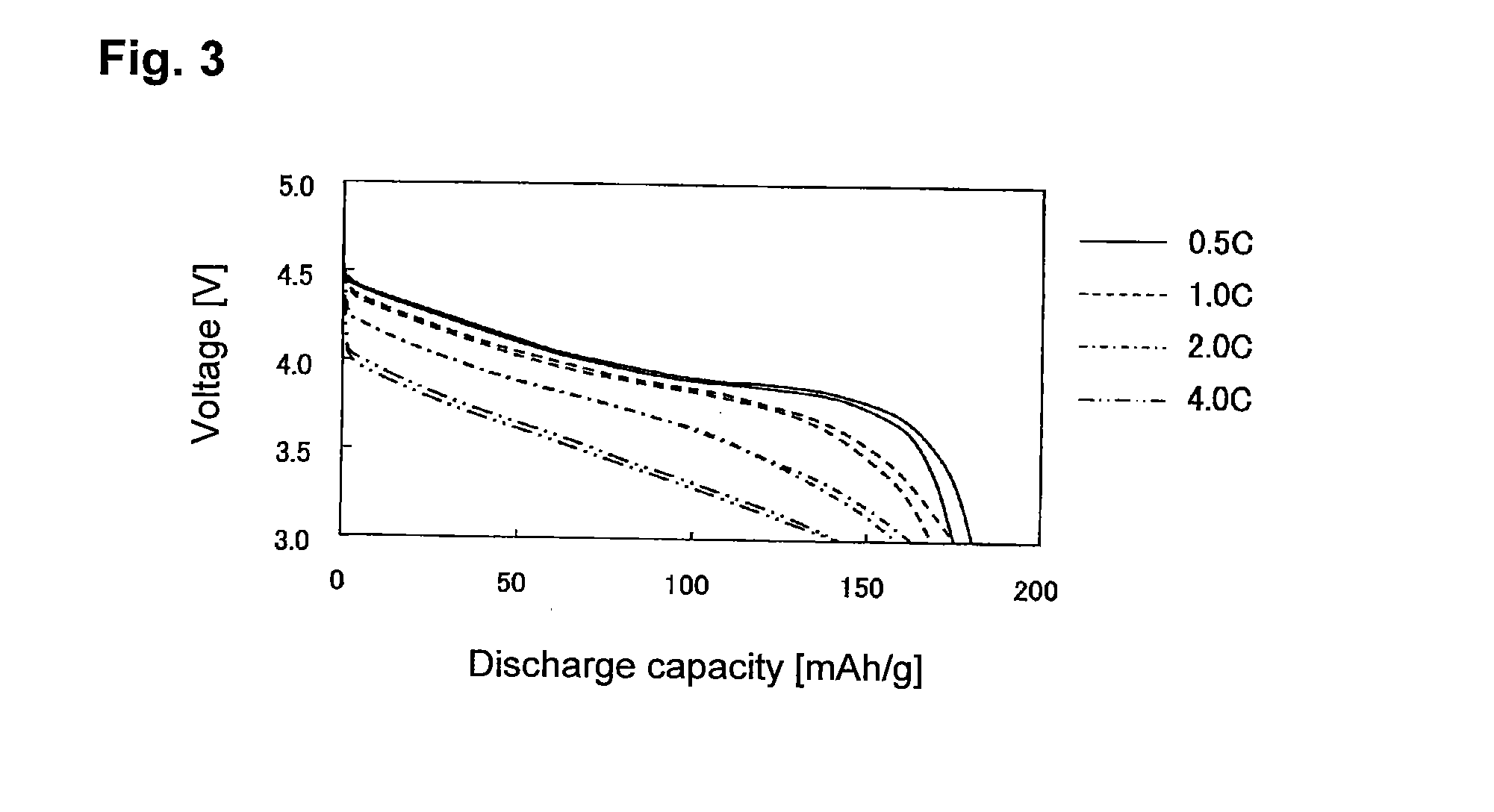
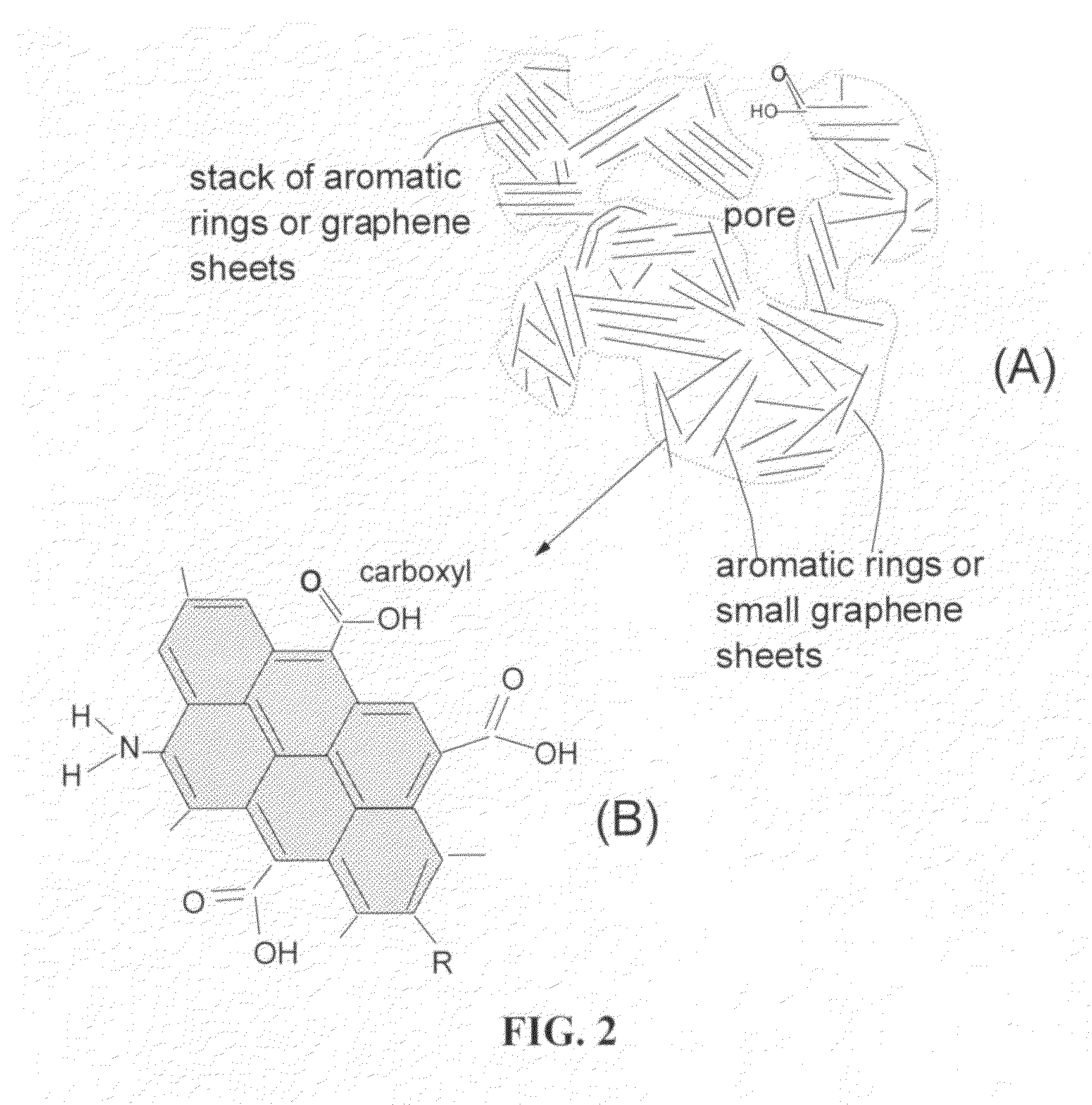
Lithium super-battery with a chemically functionalized disordered carbon cathode
ActiveUS20120077080A1Alkaline accumulatorsHybrid capacitor electrodesActivated carbonChemical functionalization
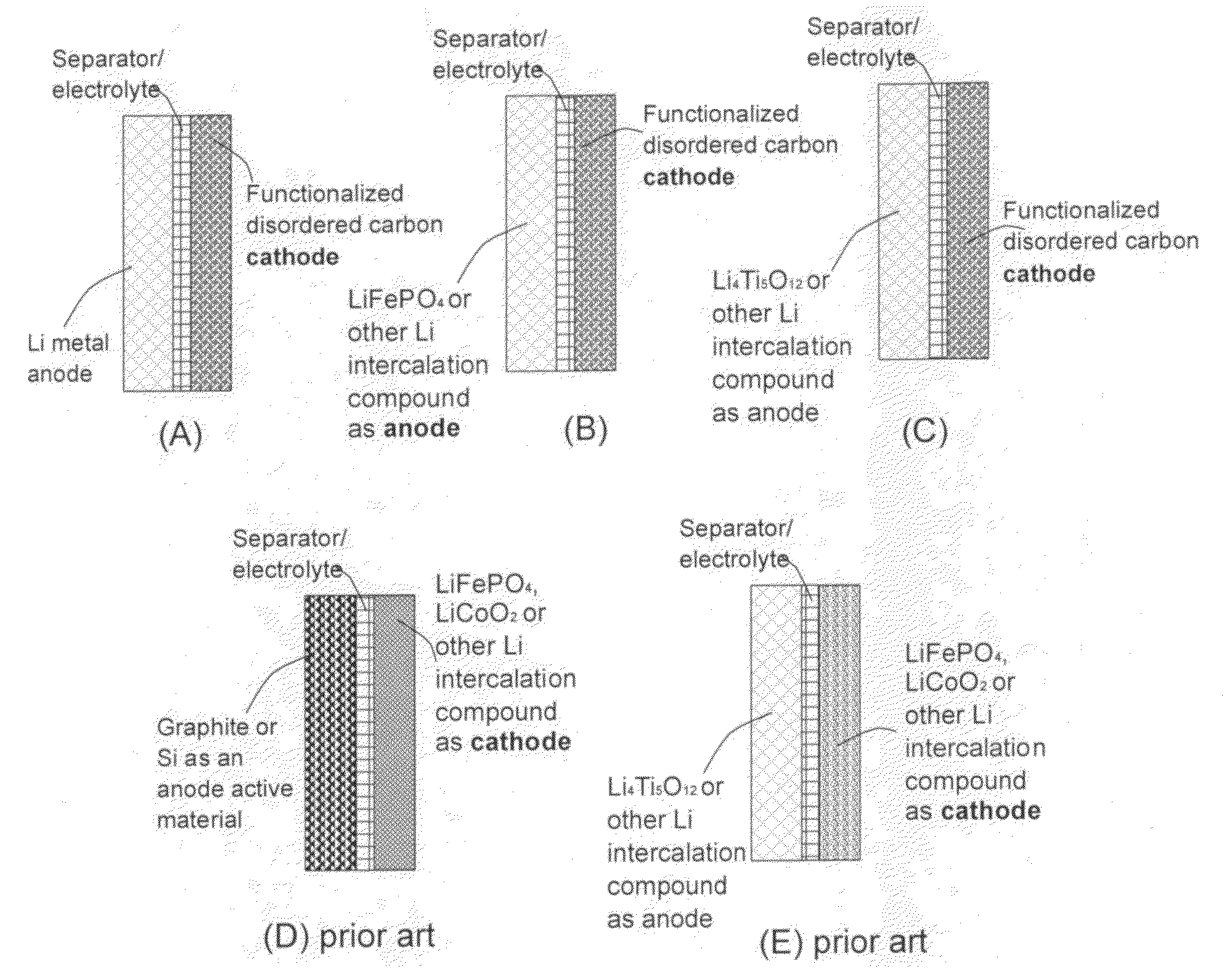
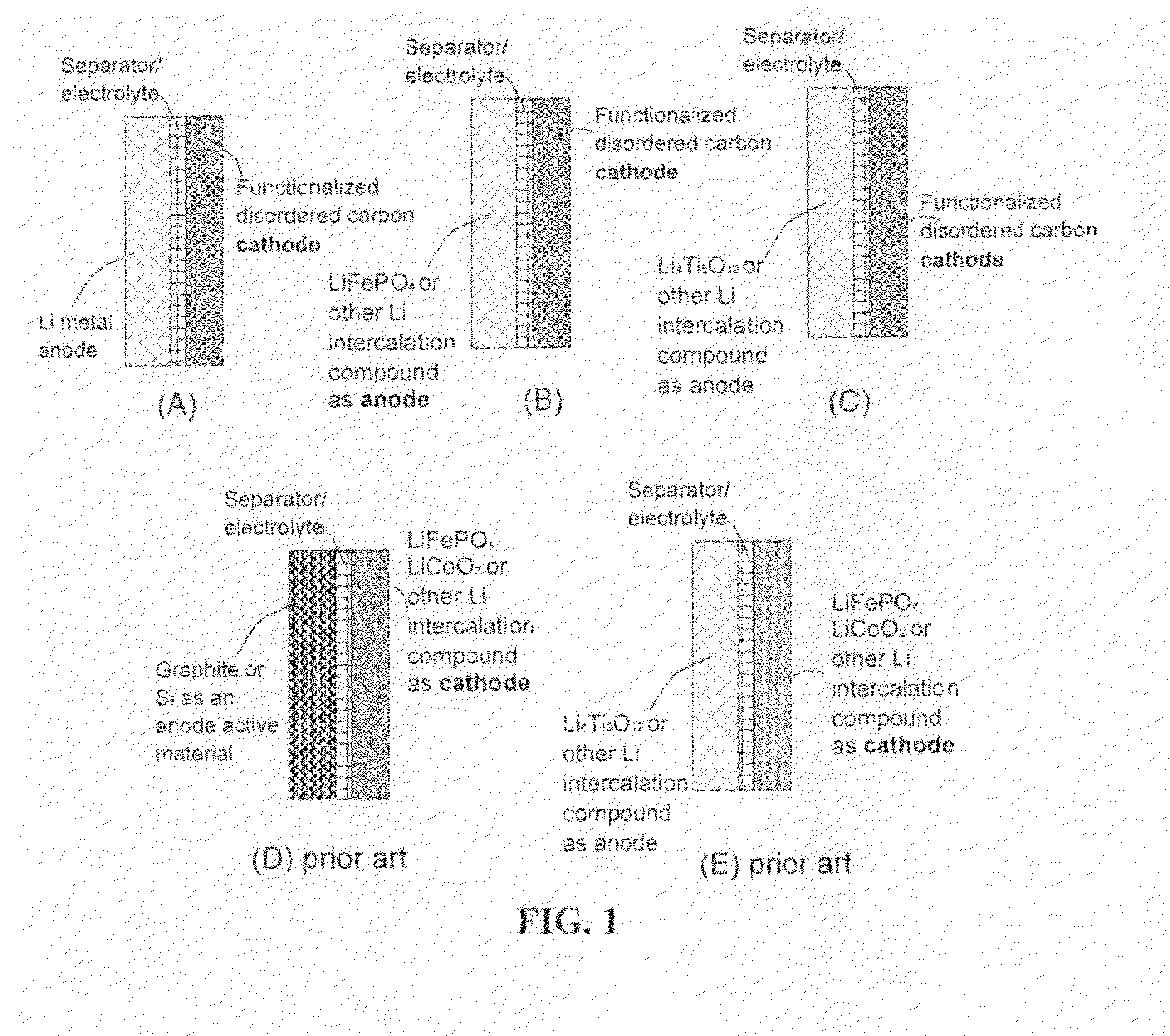
An electrochemical energy storage device, lithium super-battery, comprising a positive electrode, a negative electrode, a porous separator disposed between the two electrodes, and a lithium-containing electrolyte in physical contact with the two electrodes, wherein the positive electrode comprises a disordered carbon material having a functional group that reversibly reacts with a lithium atom or ion. The disordered carbon material is selected from a soft carbon, hard carbon, polymeric carbon or carbonized resin, meso-phase carbon, coke, carbonized pitch, carbon black, activated carbon, or partially graphitized carbon. In a preferred embodiment, a lithium super-battery having a functionalized disordered carbon cathode and a Li4Ti5O12 anode exhibits a gravimetric energy ˜5-10 times higher than those of conventional supercapacitors and a power density ˜10-30 times higher than those of conventional lithium-ion batteries. This device has the best properties of both the lithium ion battery and the supercapacitor.
Owner:NANOTEK INSTR GRP LLC +1
Aluminum/alkaline or alkali/titanium containing polyesters having improved reheat, color and clarity
ActiveUS20070066735A1Improving reheat rateIncrease ratingsHydrogenOther chemical processesPolyesterPolymer science
A polyester composition containing: a) aluminum atoms; and b) alkaline earth atoms or alkali metal atoms or alkali compound residues such as lithium atoms; and c) particles comprising titanium, zirconium, vanadium, niobium, hafnium, tantalum, chromium, tungsten, molybdenum, iron, or nickel atoms or combinations thereof, where the particles improve the reheat rate of the polyester composition. The polyester polymer compositions may also contain phosphorus catalyst deactivators / stabilizers. The polyester compositions and the articles made from the compositions such as bottle preforms and stretch blow molded bottles have improved reheat rate while maintaining low haze, high L*, a b* below 3, and have low levels of acetaldehyde. In the process for making the polyester polymer, the polymer melt is polycondensed in the presence of a) and b), with the particles c) added in a melt phase process or added to the polymer in an injection molding machine or extruder. The polyester polymer composition can be made to high IV from the melt phase while avoiding solid state polymerization.
Owner:ALPEK POLYESTER SA DE CV
Lithium secondary cell
InactiveUS6228532B1Reduce film thicknessElectrode carriers/collectorsActive material electrodesPolyelectrolyteHydrogen atom
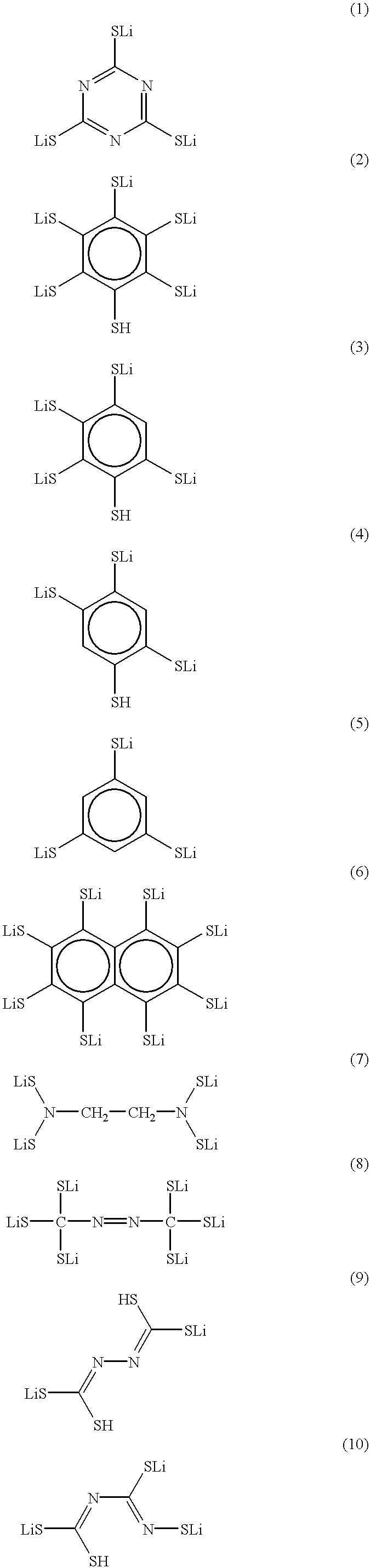
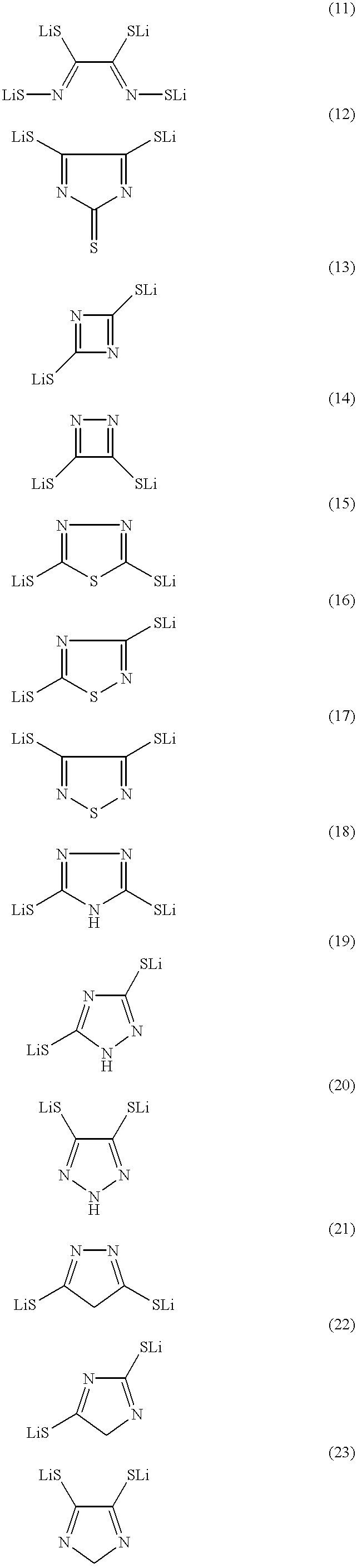
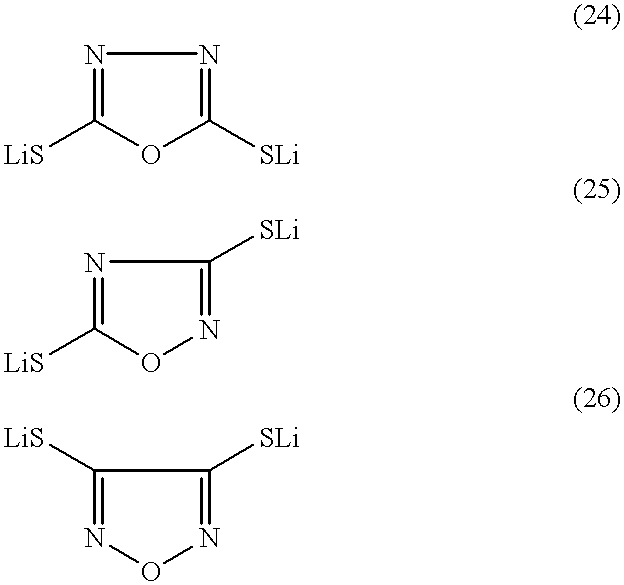
There is provided a lithium polymer secondary cell in which reduction of the film thickness of a solid electrolyte can be realized. The lithium ion secondary cell consists essentially of a composite electrode as a positive electrode having a positive electrode layer, containing a mercaptide compound and a conductive material, carried on a collector; the hydrogen atom of at least one mercapto group in the mercaptide compound being substituted with a lithium atom; a polyelectrolyte; and a lithium negative electrode.
Owner:PIONEER CORP
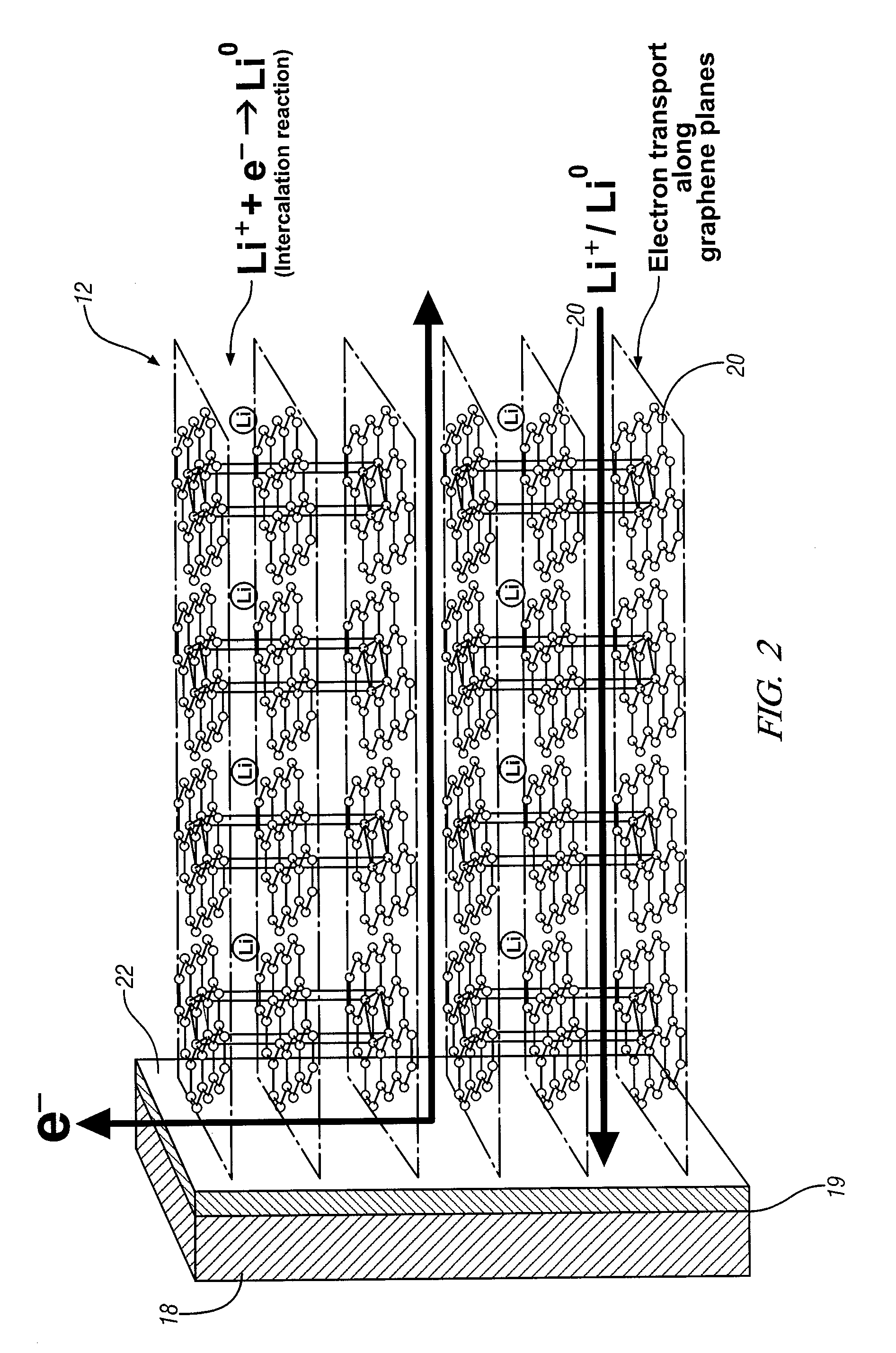
Intercalation electrode based on ordered graphene planes
ActiveUS9356281B2Efficient transportImprove volumetric efficiencySilver accumulatorsPolycrystalline material growthElectron currentGas phase
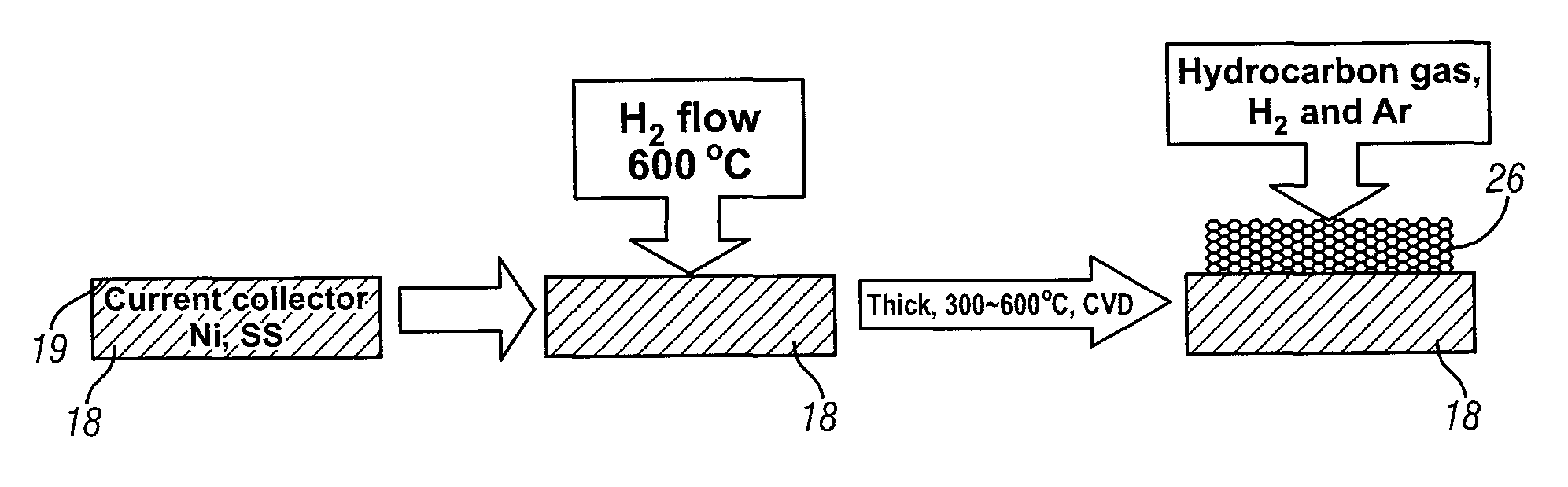
An intercalation electrode includes an electron current collector and graphene planes deposited normal to the surface of the current collector substrate. The graphene planes are deposited on the current collector substrate from a carbon-precursor gas using, for example, chemical vapor deposition. In an embodiment of an anode for a lithium-ion battery, the graphene planes are intercalated with lithium atoms. A lithium-ion battery may include this anode, a cathode, and a non-aqueous electrolyte. In repeated charging and discharging of the anode, lithium atoms and ions are readily transported between the graphene planes of the anode and the electrolyte.
Owner:GM GLOBAL TECH OPERATIONS LLC
SOLID ELECTROLYTE, METHOD FOR PRODUCING SOLID ELECTROLYTE, AND LITHIUM-ION BATTEry
A solid electrolyte includes a plurality of particles having lithium ionic conductivity and a matrix which is interposed among the particles so as to be in contact with each of the particles and is formed from an amorphous material containing the following (a) and (b): (a) lithium atoms; and (b) an oxide of at least one element selected from the group consisting of boron, a Group 14 element in period 3 or lower, and a Group 15 element in period 3 or lower.
Owner:SEIKO EPSON CORP
Lithium-site doped and metal oxide-coated lithium ion battery positive electrode material and preparation method thereof
ActiveCN104659344APerfect layered structureImprove high-current discharge performanceElectrode manufacturing processesSecondary cellsAlkaline earth metalElectrochemistry
The invention discloses a lithium-site doped and metal oxide-coated lithium ion battery positive electrode material and a preparation method thereof. The lithium ion battery positive electrode material has the chemical formula of Li(m-x)MexMaNbCcO2.yTO2, wherein Me refers to doped alkali metal or an alkaline-earth metal element; TiO2 refers to the coated metal oxide. The lithium-site doped and metal oxide coated lithium ion battery positive electrode material has the advantages that the coating metal oxide can be used for preventing the large-area contact of the lithium ion battery positive electrode material and electrolyte, and inhibiting the reaction between the battery positive electrode material with the high reaction property and electrolyte, so that the cyclic stability of the battery material can be improved greatly; the laminar structure of the lithium ion battery positive electrode material can be perfected by adopting coating, so that the electrochemical performance is also improved; partial lithium atoms in the battery positive electrode material can be replaced with alkali metal or alkaline-earth metal ions by adopting doping, so that a metal oxide layer is strutted, embedding and de-embedding of the lithium ion are facilitated, the high-current discharge performance of the material is improved, and the use of the rare element lithium is reduced.
Owner:SOUTHEAST UNIV
Lithium super-battery with a functionalized nano graphene cathode
An electrochemical energy storage device, lithium super-battery, comprising a positive electrode, a negative electrode, a porous separator disposed between the two electrodes, and a lithium-containing electrolyte in physical contact with the two electrodes, wherein the positive electrode comprises a plurality of chemically functionalized nano graphene platelets (f-NGP) or exfoliated graphite having a functional group that reversibly reacts with a lithium atom or ion. In a preferred embodiment, a lithium super-battery having a f-NGP positive electrode and Li4Ti5O12 negative electrode exhibits a gravimetric energy ˜5 times higher than conventional supercapacitors and a power density ˜10 times higher than conventional lithium-ion batteries. This device has the best properties of both the lithium ion battery and the supercapacitor.
Owner:GLOBAL GRAPHENE GRP INC +1
Battery separator and nonaqueous lithium ion secondary battery having the same
InactiveUS20100239900A1Little deteriorated in capacityAvoid depositionSolid electrolytesFinal product manufactureIminodiacetic acidHydrogen atom
The invention provides a battery separator comprising a porous resin film and a crosslinked polymer supported thereon and having iminodiacetic acid groups in side chains of the polymer chains. The iminodiacetic acid group is preferably represented by the formulawherein M1 and M2 are each independently a hydrogen atom, a lithium atom, a potassium atom, a sodium atom, or triethylamine. It is preferred that the layer of the crosslinked polymer is substantially nonporous or solid, and ion conductive, and that the crosslinked polymer has in the molecule oxetanyl groups which are capable of cation polymerization.
Owner:NITTO DENKO CORP
Composite metal lithium cathode with lithium-carbon composite interface layer and preparation method thereof
ActiveCN109686921APromote growthImprove cycle performanceCell electrodesSecondary cellsCyclic processCarbon composites
The invention discloses a composite metal lithium cathode with a lithium-carbon composite interface layer and a preparation method thereof and belongs to the technical field of secondary batteries. The outer surface of the carbon framework material of the composite metal lithium cathode is coated with the lithium-carbon composite interface layer, and the structure of the composite interface layeris a lithium-carbon intercalation structure formed by intercalating metal lithium atoms into the carbon skeleton material layer. The forming method comprises the following steps: pressing lithium metal into pores of the carbon framework material in a pressurizing mode, and forming a lithium-carbon composite interface layer, which is conductive and stable to lithium, on the surface of the carbon framework material after activation for a certain time due to the adsorption or intercalation effect. The preparation method is simple and feasible, and the generated lithium-carbon composite interfacelayer is very uniform in distribution and thickness in the carbon framework material. The interface layer can effectively improve the volume expansion problem of the lithium metal cathode in the circulation process and prolong the cycle life of the batteries.
Owner:TSINGHUA UNIV
Lamina-structure lithium-contained composite metal oxide coated with carbon and use thereof
ActiveCN1979929ALow priceImprove charge and discharge efficiencyCell electrodesLithium compoundsCarbon layerDischarge efficiency
Being as composite material with a nucleocapsid structure, the disclosed core material is in laminated structure of composite metal oxide granules containing lithium, and carbon layer as shell covers granule. Structural features of the composite metal oxide material are that oxygen atom layer, lithium atom layer, oxygen atom layer, metal layer and oxygen atom layer are arranged alternately in sequence in direction perpendicular to c axis in crystal structure. The composite metal improves surface electron conductance and electric contact so as to raise charging and discharging specific capacity and magnification performance. Features are: high charging and discharging efficiency, improved cycle performances, low cost and no pollution, high energy density of lithium cell of using the composite material as material for positive pole, safety, and applicable to multiform occasion.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
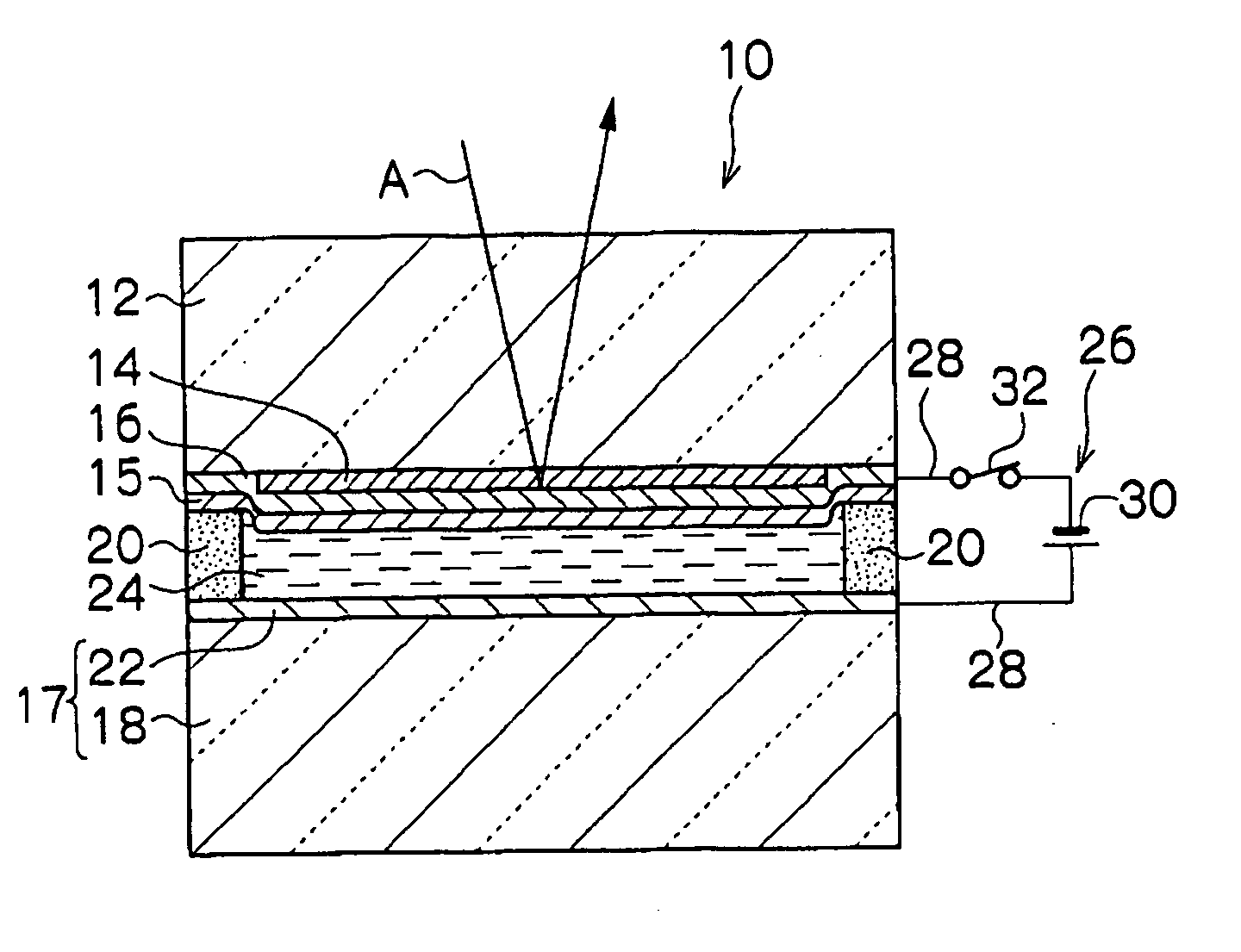
Electrochromic mirror having variable reflectivity
ActiveUS7088490B2Reduced responseEasy to manufactureNon-linear opticsOptical viewingHydrogenHydrogen atom
The present invention provides an electrochromic mirror including: a transparent substrate; a transparent electrode film which is formed on the transparent substrate and has conductivity; an electrochromic film which is formed on the transparent electrode film and can be colored by reduction; a light reflecting film which is formed on the electrochromic film and which hydrogen atoms or lithium atoms can permeate; a support substrate which has a conductive part having conductivity formed on at least one surface thereof; and an electrolytic solution containing at least hydrogen ions or lithium ions, and neutral molecules or negative ions which can be oxidized, wherein the transparent substrate is located close to the support substrate such that the conductive part faces the light reflecting film, and the electrolytic solution is enclosed between the light reflecting film and the conductive part.
Owner:KK TOKAI RIKA DENKI SEISAKUSHO
Non-aqueous electrolyte solution for secondary batteries, and secondary battery
InactiveUS20130108932A1Improve cycle performanceHigh rate charge/discharge propertiesOrganic electrolyte cellsSecondary cellsHigh rateElectrical battery
To provide a non-aqueous electrolyte solution for secondary batteries, which has a long term flame retardancy and which is excellent in cycle properties particularly under high voltage conditions and in high rate charge / discharge properties, and a secondary battery using such a non-aqueous electrolyte solution for secondary batteries. A non-aqueous electrolyte solution for secondary batteries, comprising a lithium salt and a solvent for dissolving the electrolyte salt, which comprises a specific hydrofluoroether, a specific ether compound and a specific carbonate compound, wherein the ratio (N0 / NLi) of the total number of moles (N0) of etheric oxygen atoms derived from the above ether compound to the total number of moles (NLi) of lithium atoms derived from the lithium salt, contained in the solvent for dissolving the electrolyte salt, is more than 1 and at most 6; and a secondary battery using such a non-aqueous electrolyte solution for secondary batteries.
Owner:ASAHI GLASS CO LTD
Electrode material and lithium ion battery using same
InactiveUS20140315102A1Eliminate the effects ofCell electrodesSolid electrolyte cellsPhosphorous acidSulfur
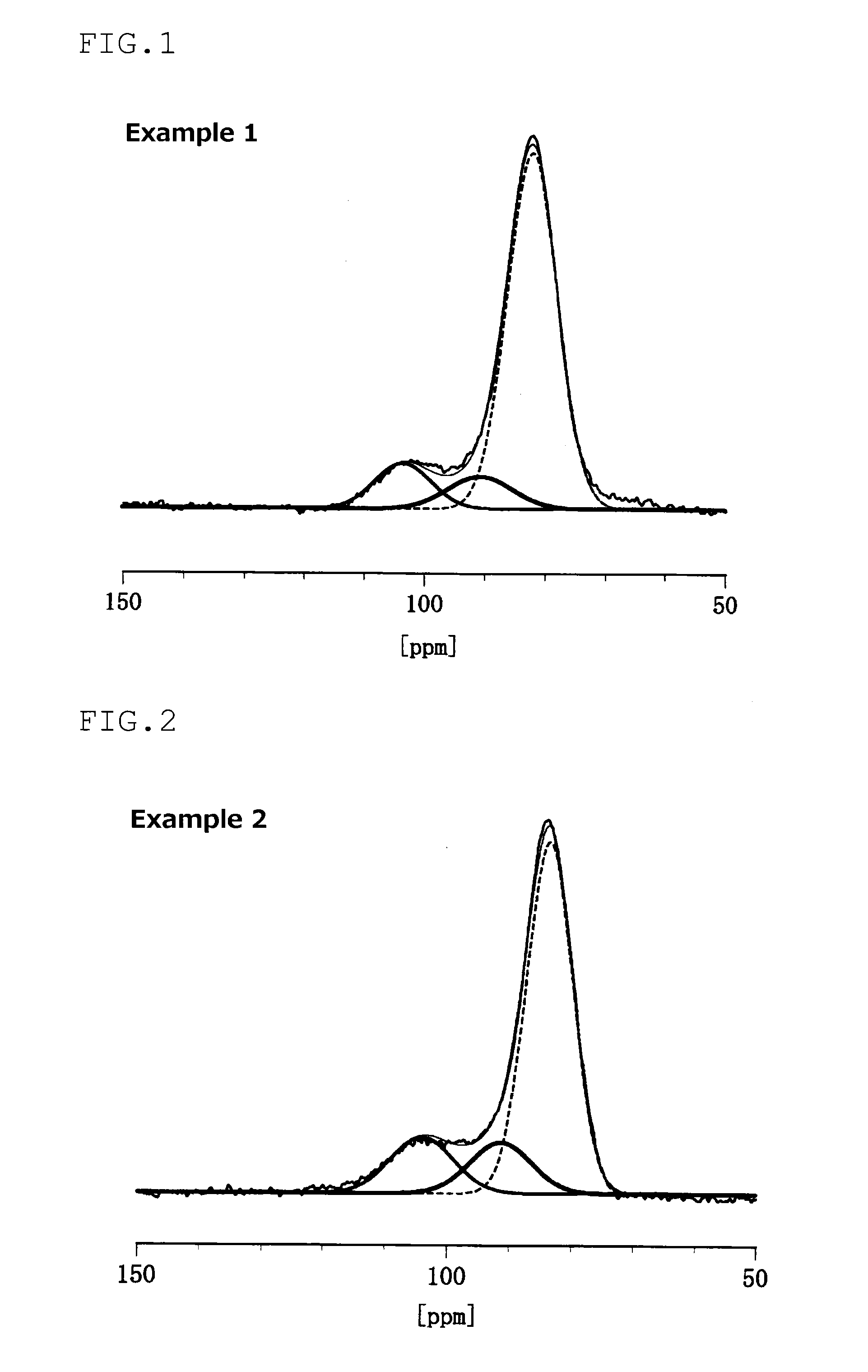
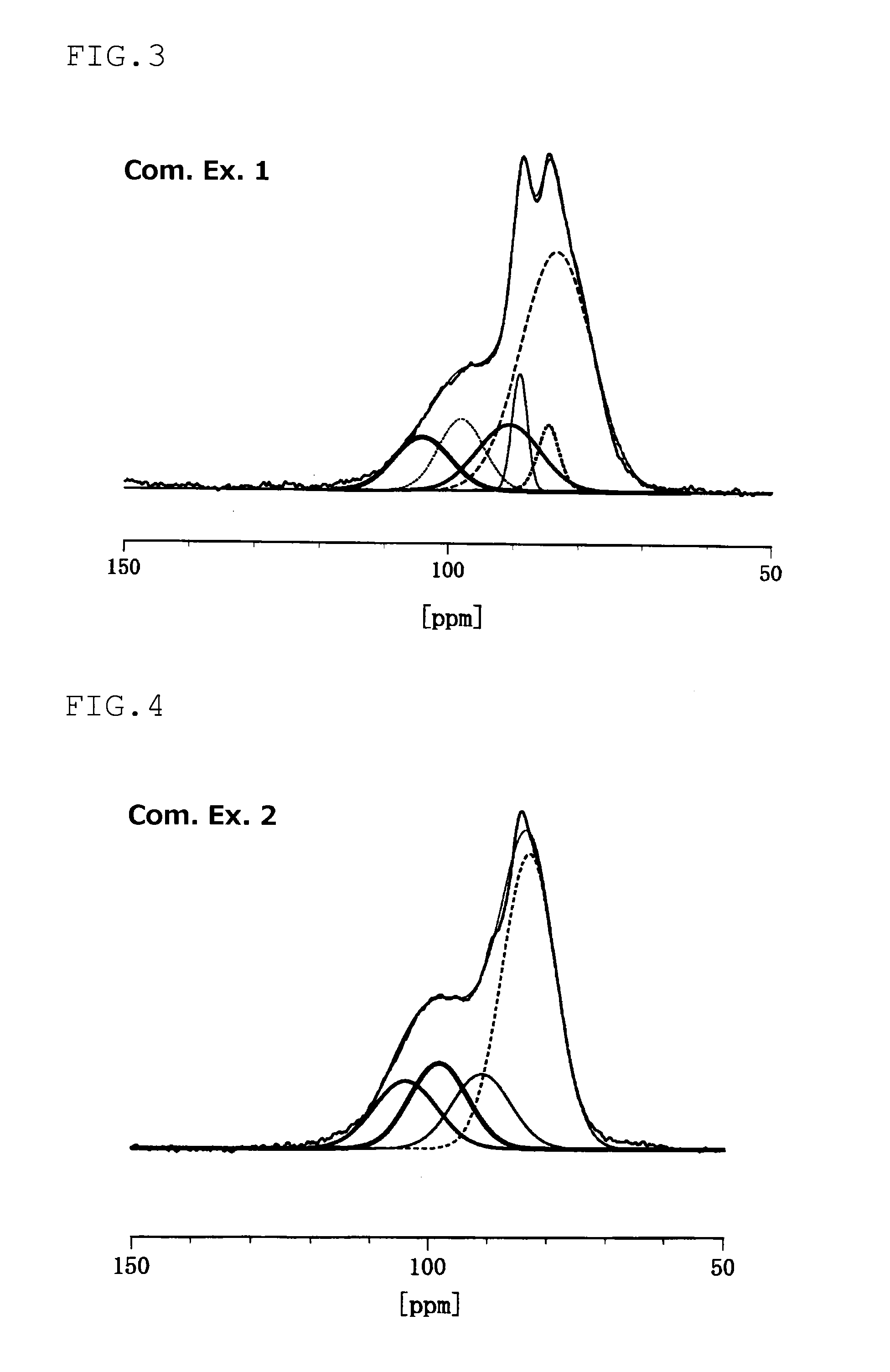
An electrode material including at least one of sulfur and a compound that contains a sulfur atom, a conductive material, and a solid electrolyte that contains a lithium atom, a phosphorous atom and a sulfur atom, wherein the solid electrolyte has at least one of a peak at 86.1±0.6 ppm and a peak at 83.0±1.0 ppm in the solid 31PNMR spectrum, and the ratio of the phosphorous atoms contained in the peak is 62 mol % or more relative to the phosphorous atoms contained in the all peaks.
Owner:IDEMITSU KOSAN CO LTD
Lithium super-battery with a chemically functionalized disordered carbon cathode
ActiveUS8900755B2Alkaline accumulatorsHybrid capacitor electrodesActivated carbonChemical functionalization
An electrochemical energy storage device, lithium super-battery, comprising a positive electrode, a negative electrode, a porous separator disposed between the two electrodes, and a lithium-containing electrolyte in physical contact with the two electrodes, wherein the positive electrode comprises a disordered carbon material having a functional group that reversibly reacts with a lithium atom or ion. The disordered carbon material is selected from a soft carbon, hard carbon, polymeric carbon or carbonized resin, meso-phase carbon, coke, carbonized pitch, carbon black, activated carbon, or partially graphitized carbon. In a preferred embodiment, a lithium super-battery having a functionalized disordered carbon cathode and a Li4Ti5O12 anode exhibits a gravimetric energy ˜5-10 times higher than those of conventional supercapacitors and a power density ˜10-30 times higher than those of conventional lithium-ion batteries. This device has the best properties of both the lithium ion battery and the supercapacitor.
Owner:NANOTEK INSTR GRP LLC +1
Solid electrolyte material including sulfide layer and oxide layer, and battery including the solid electrolyte material
ActiveUS20180316057A1Reduce generationLow efficiencySolid electrolytesCell electrodesSulfurPhysical chemistry
A solid electrolyte material includes: a sulfide layer containing lithium atoms and sulfur atoms; and an oxide layer covering the sulfide layer, the oxide layer containing lithium atoms and oxygen atoms. The solid electrolyte material satisfies 0.51≤x and x / y≤1.53, where x is a first ratio of the number of the oxygen atoms to the number of the lithium atoms at a depth 4 nm of the solid electrolyte material from the surface of the oxide layer; and y is a second ratio of the number of the oxygen atoms to the number of the lithium atoms at a depth 100 nm of the solid electrolyte material from the surface of the oxide layer.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Fullerene based hydrogen storage system
A hydrogen storage structure includes a plurality of graphene sheets arranged to form a stack with a plurality of spacers between adjacent graphene sheets in the stack. In one embodiment, the spacers are arranged to provide a distance ranging between 5 Å and 20 Å between adjacent graphene sheets. In one embodiment, the spacers are formed as graphene spheres having a diameter that ranges from 5 Å to 15 Å. In another embodiment, the spacers are formed as graphene single-walled nanontubes having a length that ranges from 5 Å to 20 Å. In a further embodiment, the spacers are formed as graphene sheets having a thickness that ranges from 5 Å to 20 Å. In one embodiment, the plurality of graphene sheets is doped with lithium. In one embodiment, the lithium doping concentration is a ratio of one lithium atom per three carbon atoms.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Preparation method of layered lithium-enriched manganese-based material Li1.2Ni0.13Co0.13Mn0.54O2
InactiveCN107123793AHigh specific capacityImprove cycle lifeMaterial nanotechnologyCell electrodesManganeseLithium-ion battery
The invention discloses a preparation method of a layered lithium-enriched manganese-based material Li1.2Ni0.13Co0.13Mn0.54O2, and belongs to the field of lithium ion battery electrode materials. The preparation method is a kilogram-grade engineering chain method, and one reaction kettle can prepare a kilogram-grade lithium-enriched manganese positive electrode material precursor at one time. The method comprises the following steps: taking cheap weak acid sodium salt as a precipitator, adjusting the pH by industrial grade alkaline hydroxide, providing Ni, Co and Mn lithium-intercalated matrixes by a transition metal salt solution and preparing a precursor [Ni0.13Co0.13Mn0.54]CO2 by a coprecipitation method, wherein the ratio of the precursor [Ni0.13Co0.13Mn0.54]CO2 to a lithium source according to the quantity of the substances is that M:Li is equal to 1:(1.2-1.5), M is the sum of the quantity of the substances such as Ni, Co and Mn, and the lithium atoms are 3 to 8 percent excessively; and after performing planetary ball milling and mixing, performing solid-phase reaction at high temperature of 750 to 950 DEG C for 12 to 18 hours to prepare the layered lithium-enriched manganese-based material Li1.2Ni0.13Co0.13Mn0.54O2 with high capacity and long life. The Li1.2Ni0.13Co0.13Mn0.54O2 material prepared by the method can solve the problem that the existing layered lithium-enriched material has low cycle performance.
Owner:JIANGSU UNIV
Preparation method of lithium iron phosphate-lithium vanadium phosphate flaky composite cathode material
ActiveCN106450298AImprove conductivityImprove ionic conductivityCell electrodesSecondary cellsNon oxidativeHigh pressure
The invention discloses a preparation method of a lithium iron phosphate-lithium vanadium phosphate flaky composite cathode material. The preparation method comprises the following steps that 1, a vanadium source, an iron source, a phosphorus source, a lithium source and an organic carbon source are dissolved into deionized water according to the molar ratio 2:1:4:4:(3-5) of vanadium atoms to iron atoms to phosphorus atoms to lithium atoms to carbon atoms, a surfactant is added, the pH value is regulated, and stirring is conducted; 2, the mixture is transferred into a high-pressure reaction kettle, protective gas is introduced, reacting is conducted for 10 h to 30 h at the speed of 200 rpm to 1,200 rpm at the temperature of 200 DEG C to 300 DEG C, and after washing, filtering and drying are conducted, grinding is conducted; 3, roasting is conducted in a non-oxidative atmosphere at the temperature of 600 DEG C to 800 DEG C, and then the material is obtained. The lithium iron phosphate-lithium vanadium phosphate flaky composite cathode material synthesized through the method has the excellent electronic conductivity and ionic conductivity and is excellent in electrochemical performance, good and stable in product homogeneity and low in cost.
Owner:CENT SOUTH UNIV
Preparation method of positive active material hollow spherical lithium manganate of lithium ion battery
The invention relates to a preparation method of positive active material hollow spherical lithium manganate of a lithium ion battery, and belongs to the technical field of a chemical battery. The method comprises the steps of uniformly mixing a manganese sulfate aqueous solution with a potassium persulfate aqueous solution, adding concentrated sulfuric acid, carrying out hydrothermal reaction, then centrifuging the mixed solution, taking out solid phase, and washing and drying the solid phase to obtain spinous hollow manganese dioxide spheres; mixing soluble lithium salt and the spinous hollow manganese dioxide spheres to obtain a mixture I, ultrasonically processing, drying and burning the mixture I to obtain hollow spherical lithium manganate. The prepared spherical lithium manganate belongs to a spinel type, the diameter of the spherical lithium manganate is 0.5 to 5 micrometers, the degree of crystallinity is good, the spherical lithium manganate is mainly formed by clustering and assembling spinous nanowires, a large clearance is formed between two adjacent nanowires, a porous structure is formed inside the spherical lithium manganate, the lithium atom density is reasonable, the activity is low, the chemical stability is high, adequate contact when the lithium salt is dissolved and the lithium manganate is synthesized in the later period can be facilitated, the insertion and separation of lithium ions can be facilitated, and the electrochemical cycling reversibility and stability of the lithium ion battery can be effectively improved.
Owner:YANGZHOU UNIV
Non-aqueous electrolyte secondary battery, positive electrode active material and method of manufacturing the same
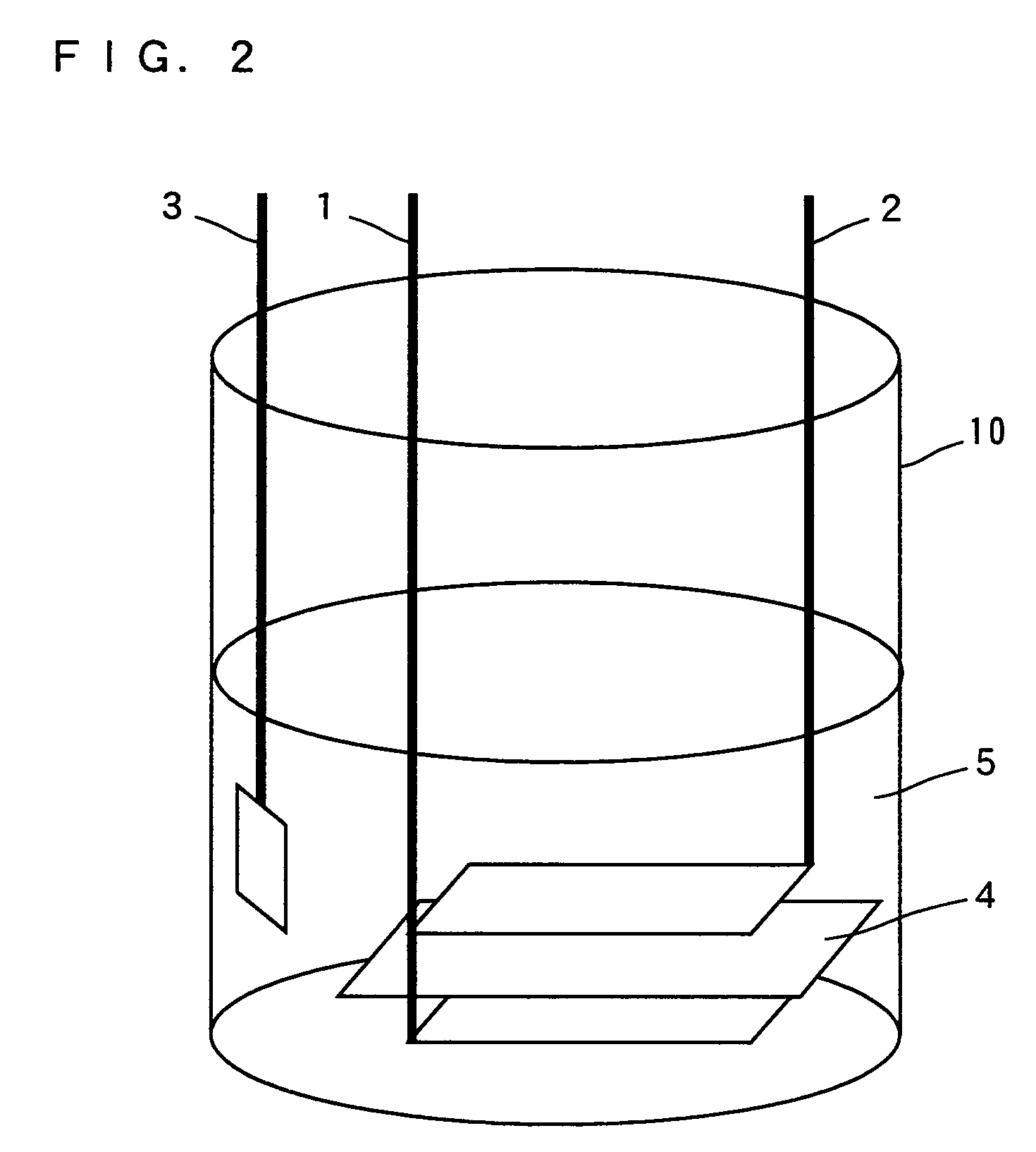
ActiveUS7709151B2Increase capacityElectrochemical processing of electrodesNon-aqueous electrolyte accumulatorsLithium atomMetal
As a positive electrode active material, a lithium transition metal complex oxide having a layered rock-salt structure containing lithium (Li) and containing magnesium atoms (Mg) substituted for part of lithium atoms (Li) is used. The lithium transition metal complex oxide is formed by chemical or electrochemical substitution of Mg atoms for part of Li atoms in LiCoO2, LiMnO2, LiFeO2, LiNiO2, or the like. A cell is prepared in which a negative electrode 2 and a positive electrode 1 including the lithium transition metal complex oxide (positive electrode active material) are disposed in a non-aqueous electrolyte 5 including a lithium salt, and part of Li in the lithium transition metal complex oxide is extracted by discharging the cell. Then, the electrolyte including Li is replaced with an electrolyte including Mg, and the cell is discharged, so that Mg atoms are substituted for the part of Li atoms in the lithium transition metal complex oxide.
Owner:SANYO ELECTRIC CO LTD
Preparation method of spherical lithium metatitanate solid tritium fertile material
InactiveCN103588476AReduce manufacturing costSimple equipment requirementsNuclear fusionCrystallinity
The invention relates to a preparation method of a spherical lithium metatitanate solid tritium fertile material, which belongs to the technical field of nuclear fusion. The method comprises the following steps: preparing titanium dioxide balls, mixing an aqueous solution of soluble lithium salt and titanium dioxide balls for performing ultrasonic treatment, then reacting, centrifuging after completing the reaction, taking a solid phase for washing, and drying and calcining to obtain spherical lithium metatitanate. The preparation method has the advantages that a reactant ethanol can be recovered and repeatedly used, the preparation cost is low, the equipment requirement is simple, and the preparation period is short. The prepared spherical lithium metatitanate belongs to a monoclinic crystal type, the diameter is 200nm to 400nm, structure is uniform, the crystallization degree is good, a pore structure is formed in the inner part, the prepared spherical lithium metatitanate has reasonable lithium atom concentration, low activity and high chemical stability, and has good compatibility with a combination material, and the spherical lithium metatitanate has good thermal stress and fission strength due to complex geometry spherical morphology.
Owner:YANGZHOU UNIV
Electrode materials for electrochemical cells
InactiveUS7629080B1Positive electrodesNon-aqueous electrolyte accumulator electrodesPhosphateDivalent metal ions
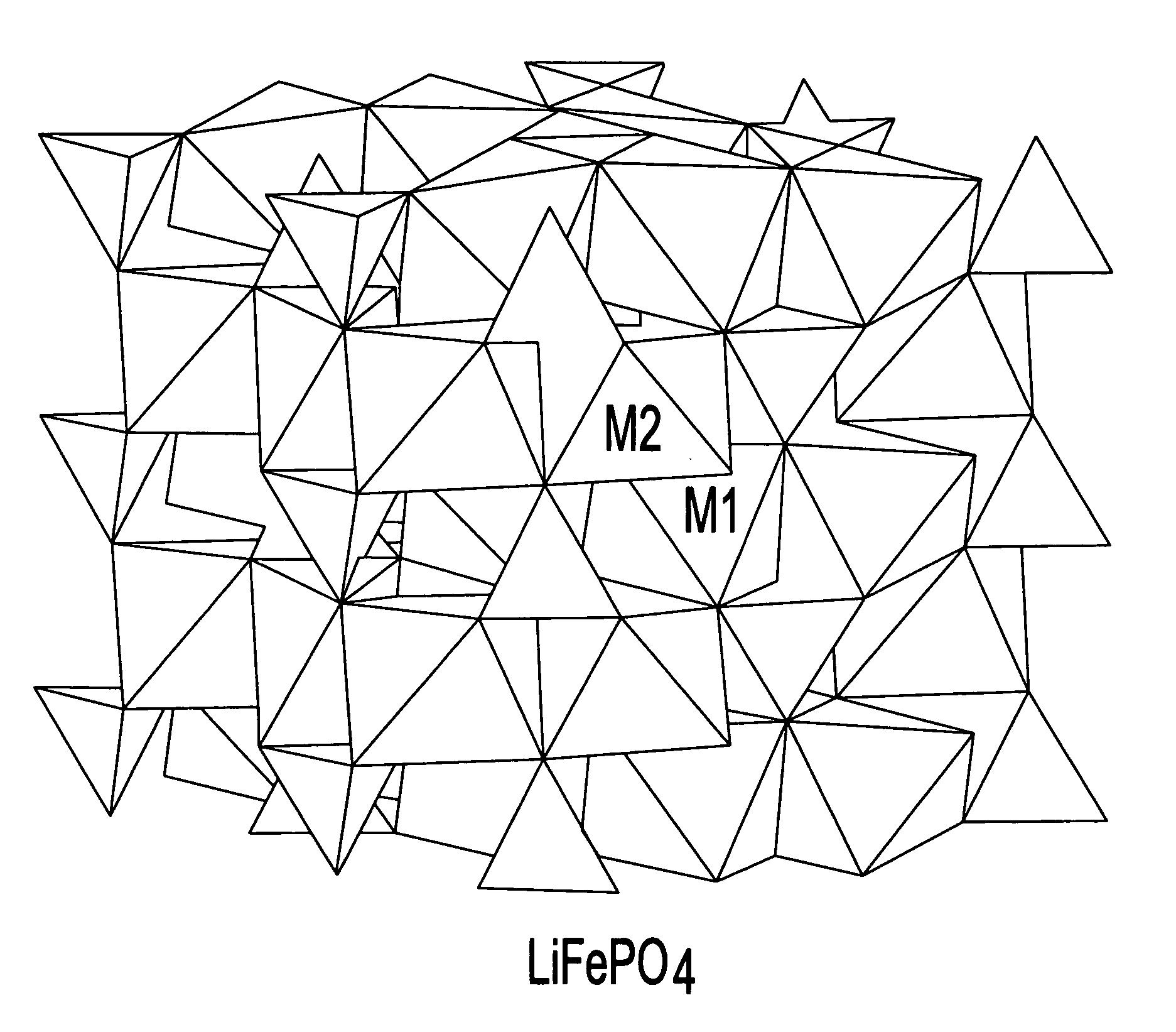
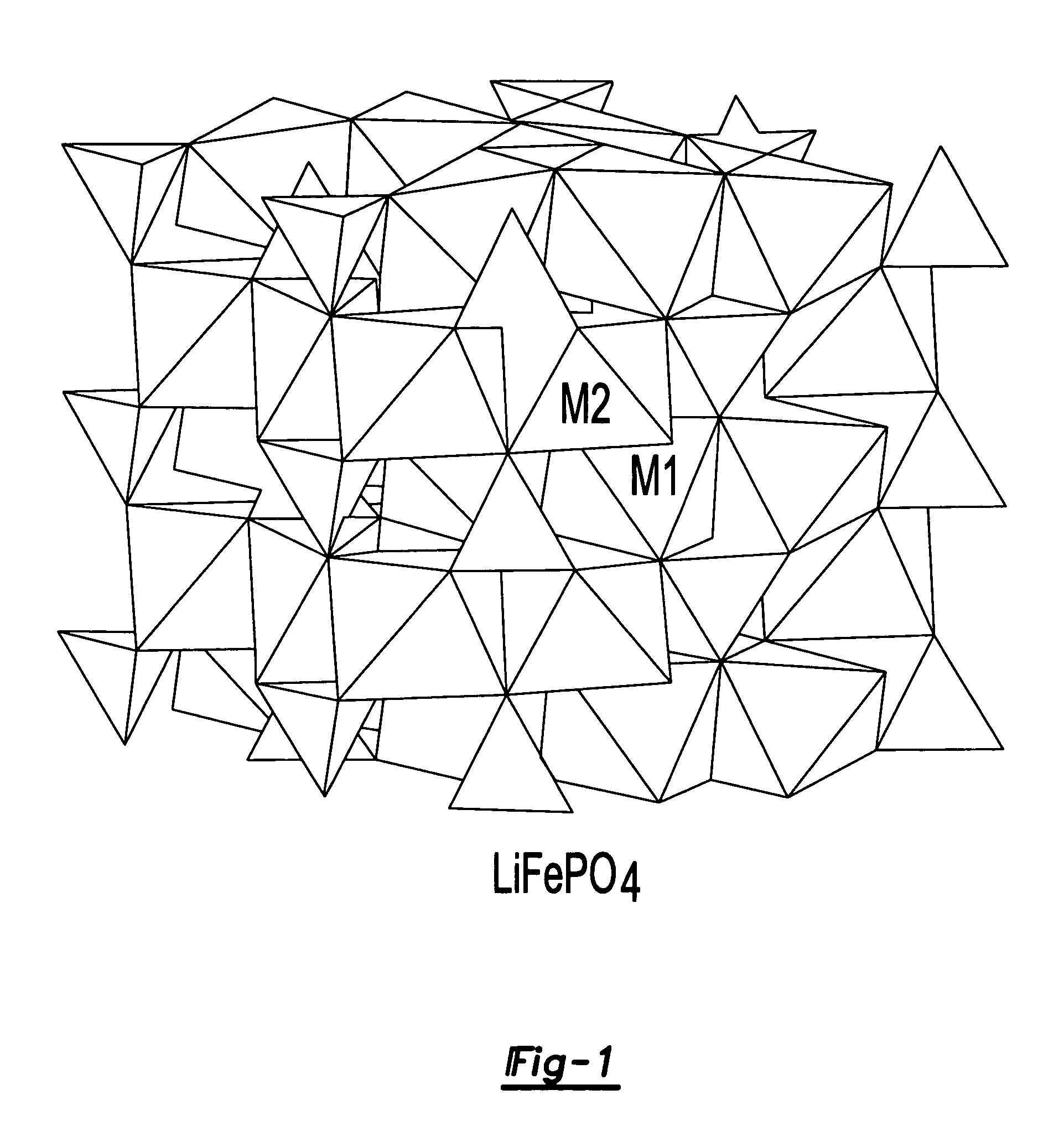
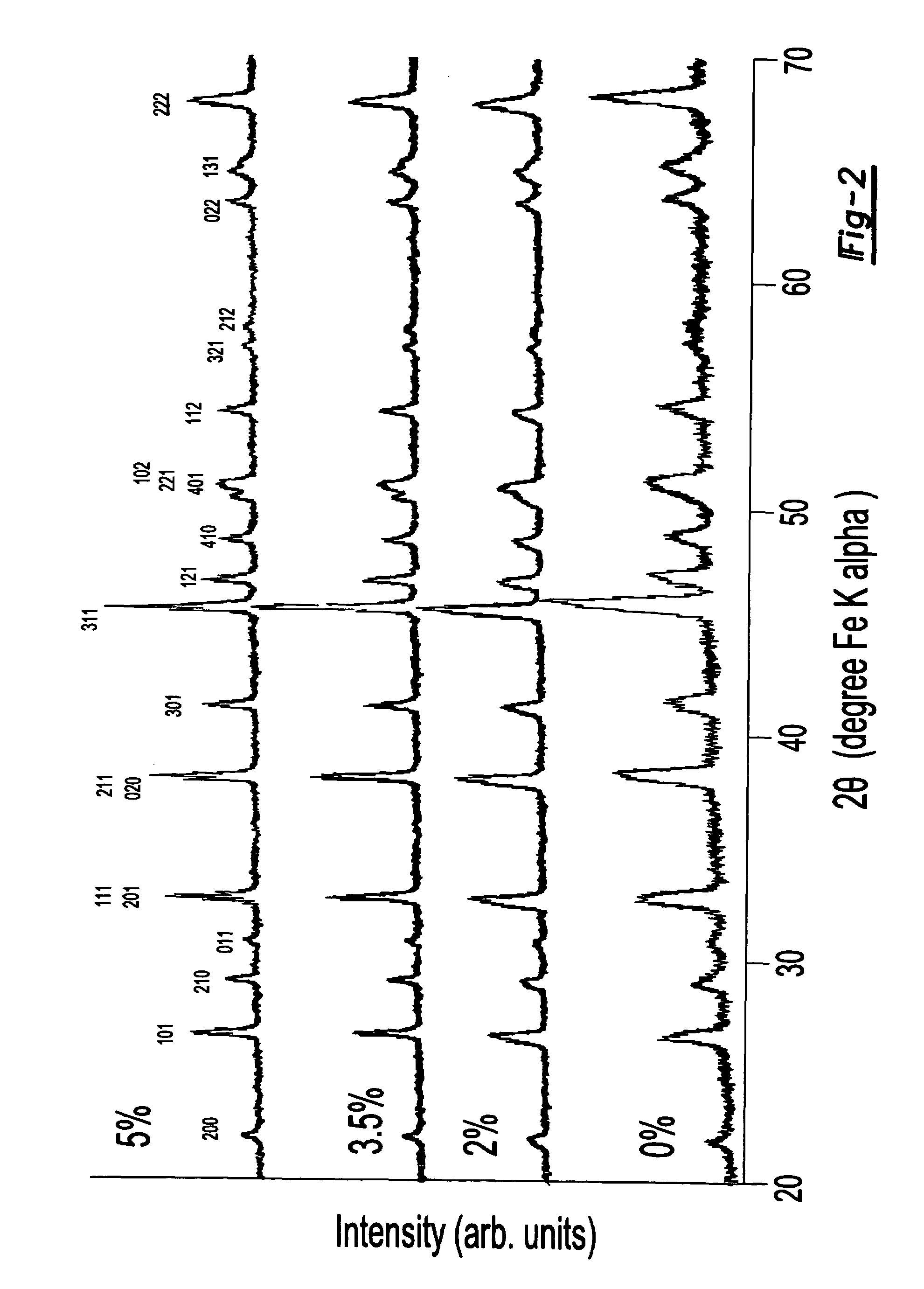
A lithiated metal phosphate material is doped by a portion of the lithium atoms which are present at the M2 sites of the material. The doped material has the general formula: Li1+xM1−x−dDdPO4. In the formula, M is a divalent ion of one or more of Fe, Mn, Co and Ni. D is a divalent metal ion which is one or more of Mg, Ca, Zn, and Ti. It is present in an amount represented by the subscript d which has a value ranging from 0 to 0.1. The portion of the lithium which is present at the M2 octahedral sites of the material is represented by the subscript x and is greater than 0 and no more than 0.07. Also disclosed are electrodes which incorporate the material as well as batteries, including lithium ion batteries, which include cathodes fabricated from the doped, lithiated metal phosphate materials.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Battery separator and nonaqueous lithium ion secondary battery having the same
InactiveUS8349031B2Little deteriorated in capacityAvoid depositionSolid electrolytesFinal product manufactureHydrogen atomSide chain
The invention provides a battery separator comprising a porous resin film and a crosslinked polymer supported thereon and having iminodiacetic acid groups in side chains of the polymer chains. The iminodiacetic acid group is preferably represented by the formulawherein M1 and M2 are each independently a hydrogen atom, a lithium atom, a potassium atom, a sodium atom, or triethylamine. It is preferred that the layer of the crosslinked polymer is substantially nonporous or solid, and ion conductive, and that the crosslinked polymer has in the molecule oxetanyl groups which are capable of cation polymerization.
Owner:NITTO DENKO CORP
Electrochromic mirror
The present invention provides an electrochromic mirror, comprising a transparent substrate; an electrochromic film that develops color reductively having the transparent substrate on or above the surface thereof; a light-reflecting film allowing transmission of hydrogen or lithium atoms formed on the side of the electrochromic film opposite to the transparent substrate; a substrate having an electrically conductive area at least on one surface facing the light-reflecting film; and an electrolyte solution at least containing a hydrogen ion and an oxidizable material containing neutral molecules or negative ions and that is sealed between the light-reflecting film and the conductive area of the substrate; wherein (i) the light-reflecting film has electrical conductivity, or (ii) a transparent electrode film having electrical conductivity is formed between the transparent substrate and the electrochromic film, further comprising a dielectric film having a hydrogen or lithium ion conductivity formed between the light-reflecting film and the electrolyte solution.
Owner:KK TOKAI RIKA DENKI SEISAKUSHO
Non-aqueous electrolytes for lithium electrochemical cells
InactiveUS20090269676A1Improve stabilityPrevent further degradationAlkaline accumulatorsConductive materialHydrogen atomHydrogen
A non-aqueous electrolyte for an electric current producing electrochemical cell is provided comprising an ionically conductive salt and an additional ionically conducting salt in a non-aqueous medium, the additional ionically conducting salt corresponding to the formula M+(Z*(J*)j)−, wherein: M is a lithium atom, Z* is an anion group containing two or more Lewis basic sites and comprising less than 50 atoms not including hydrogen atoms, J* independently each occurrence is a Lewis acid coordinated to at least one Lewis basic site of Z*, and optionally two or more such J* groups may be joined together in a moiety having multiple Lewis acidic functionality, and j is an integer from 2 to 12. The addition of these ionically conducting salts to electrolyte solutions containing LiPF6 (and / or other lithium compounds) improves the stability of the electrolyte solution.
Owner:MYSTICMD
Low-chromium cast iron grinding ball and casting method thereof
The invention relates to a low-chromium cast iron grinding ball and a casting method thereof. The low-chromium cast iron grinding ball contains carbon, silicon, manganese, chromium, nickel, molybdenum, copper, boron, lithium, sodium, rare-earth elements and essential impurity elements. The casting method comprises the following steps: preparing raw materials and an evaporative mold according to requirements, adding a simple substance sodium into the evaporative mold, and casting molten metal into the evaporative mold; and after the casting is cooled to 300-520 DEG C, taking out the casting, and carrying water quenching to finish the casting. The small-atom lithium and sodium are added, so that the sodium atoms and lithium atoms are diffused and filled in clearances of eutectic carbides or pores in the crystalline phase in the cast iron under high-temperature conditions, thereby greatly lowering the number of internal defects; and the boron powder and rare-earth elements can increase the quantity of the eutectic structures and enhance the dispersivity, so that the lithium atoms and sodium atoms can be filled and distributed in the whole structure, thereby enhancing the overall performance.
Owner:王会智
Non-aqueous electrolyte solution for secondary batteries, and lithium ion secondary battery
ActiveUS20140322616A1Reduced responseImprove cycle performanceOrganic electrolyte cellsSecondary cellsPhysical chemistryCarboxylic acid
A non-aqueous electrolyte solution for secondary batteries, comprising a lithium salt (total number of moles of lithium atoms: NLi) and a liquid composition, wherein the liquid composition comprises a specific fluorinated solvent (α) and a cyclic carboxylic acid ester compound (total number of moles: NA), and may contain a specific compound (β) (total number of moles: NB), the content of the fluorinated solvent (α) is from 40 to 80 mass %, NA / NLi is from 1.5 to 7.0, and (NA+NB) / NLi is from 3 to 7.0; and, a lithium ion secondary battery employing such a non-aqueous electrolyte solution for secondary batteries.
Owner:ASAHI GLASS CO LTD
Preparation method of lithium difluoro(oxalato)borate
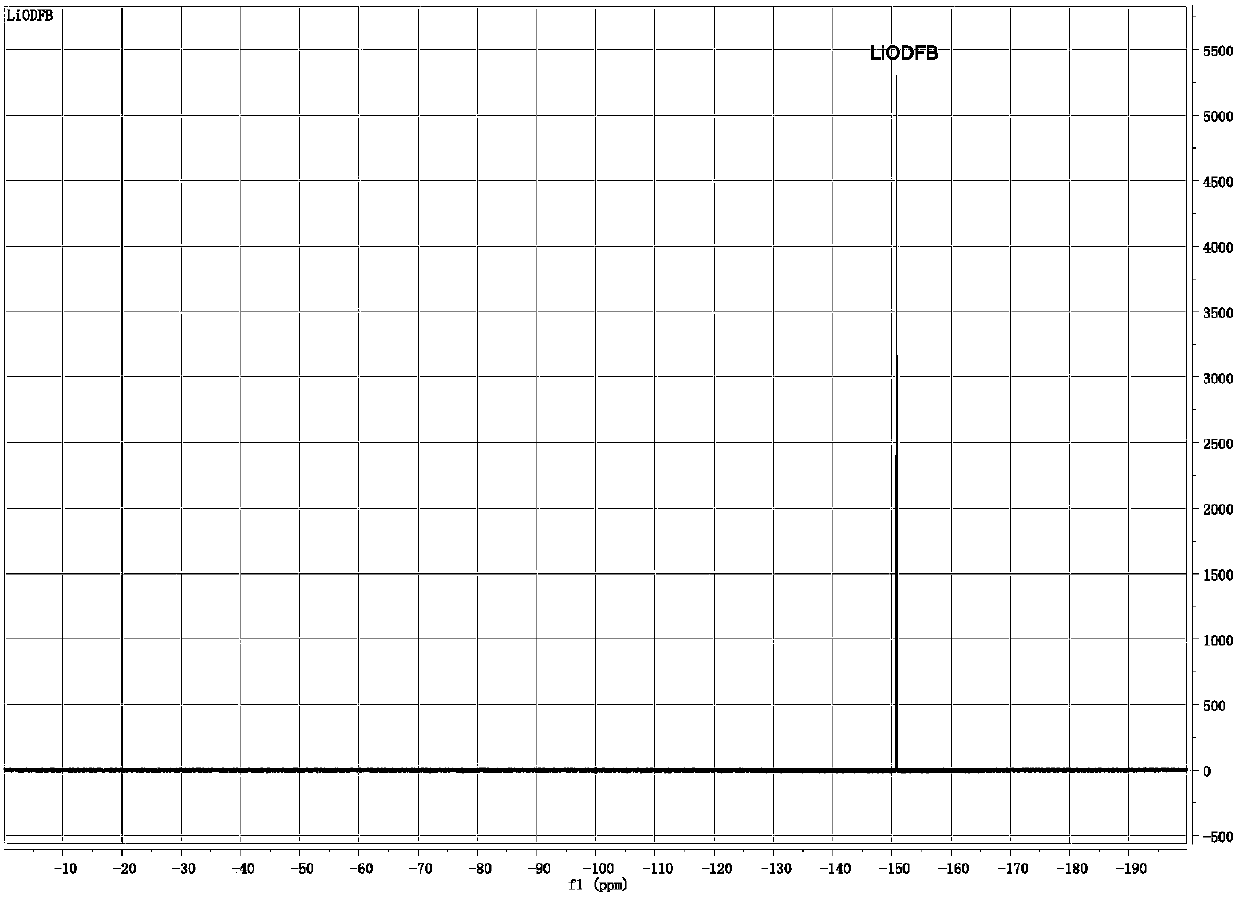
The invention provides a preparation method of lithium difluoro(oxalato)borate (Li[B(C2O4)F2], LiDFOB). The method effectively recycles the by-product LiBF4, and avoids the waste of lithium atoms while improving the yield. The method utilizes the solubility difference of materials to directly filter out the by-product, and is simple in operation. The crude product is subjected to crystallization directly to obtain high purity LiDFOB, thus avoiding the product loss brought about by repeated recrystallization. The method has the advantages of cheap and easily available materials, simple reactionsteps and mild reaction conditions, and is suitable for large-scale industrial production.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com