Preparation method of layered lithium-enriched manganese-based material Li1.2Ni0.13Co0.13Mn0.54O2
A lithium-rich manganese-based, layered technology, applied in nanotechnology, electrical components, electrochemical generators for materials and surface science, etc., can solve the problems of memory effect, high price, high toxicity, etc., and achieve good cycle Longevity and rate performance, improved specific capacity, good consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
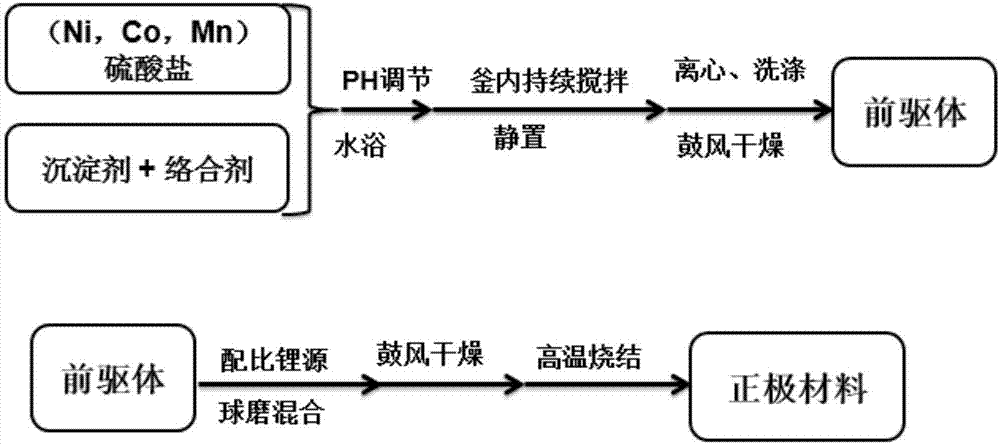
[0036] (1) Co-precipitation steps to prepare nickel-cobalt-manganese carbonate precursor:
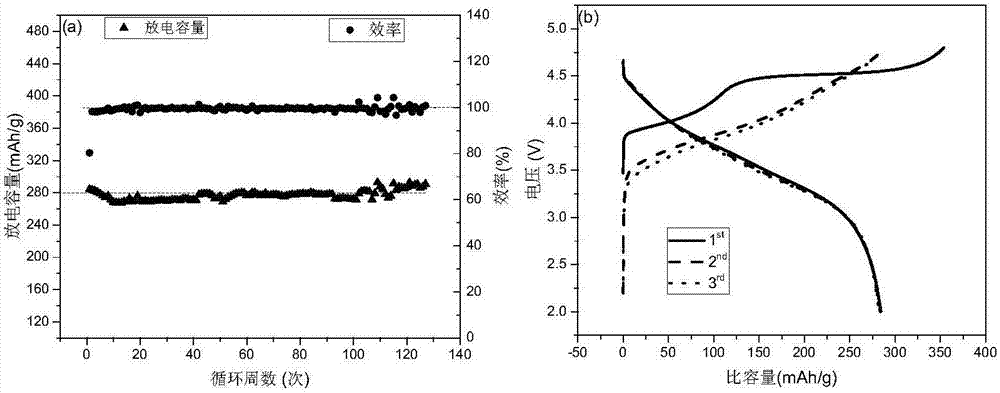
[0037] Such as figure 1 , nickel sulfate, cobalt sulfate, manganese sulfate are formulated into a uniform solution of 1mol / L according to the metal ion ratio Ni:Co:Mn=1:1:4 of the prepared material, and a sufficient amount of sodium carbonate is weighed to be mixed with 2mol / L The solution is used as a precipitating agent, and simultaneously, saturated ammonia is added to the sodium carbonate solution to form a 0.3mol / L ammonia complexing agent. The concentration of the bottom liquid in the reactor is 0.167mol / L, and the ratio of nickel, cobalt, manganese metal ions is the same as that of the feed liquid. The solution was continuously added to the reactor at a rate of 2 mL / min, and the reaction continued for 18 hours. Such as Figure 4 (a), (b), (c) are the SEM images of the precursor. After the reaction, the precursor in the kettle was left to stand for 2 hours, and then the precur...
Embodiment 2
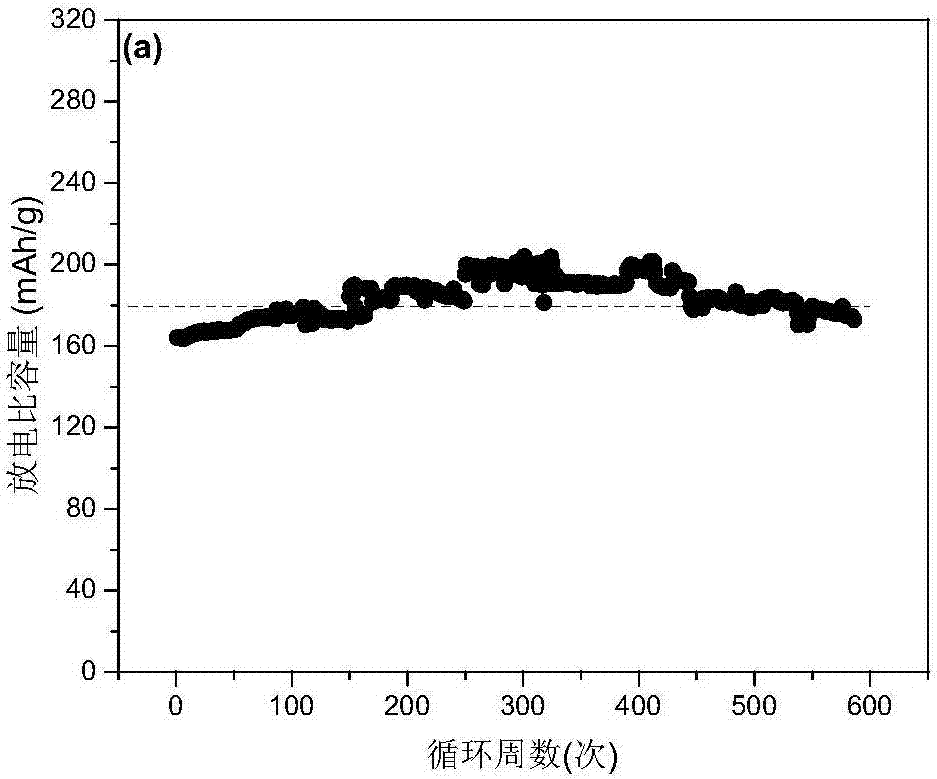
[0044] The stirring speed was adjusted to 600rpm, and the remaining reaction conditions and experimental methods were exactly the same as in Example 1. Li prepared in this example 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 Electrochemical performance curves of layered lithium-rich manganese-based materials, such as Figure 5 shown. Figure 5 It is the 1C discharge cycle performance of the lithium-rich manganese-based positive electrode material of Example 2 and the charge-discharge curves of the first three weeks. It can be seen from the figure that the capacity of the material after 550 cycle charge-discharge cycles is basically unchanged compared with the initial discharge capacity, indicating that the material It has good high-rate cycle stability, but the specific capacity has been around 160mAh / g, which is not very high.
Embodiment 3
[0046] The pH of the reaction solution was adjusted to 8.5, and the remaining reaction conditions and experimental methods were exactly the same as in Example 1. Li prepared in this example 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 Electrochemical performance curves of layered lithium-rich manganese-based materials, such as Figure 6 shown. Figure 6 It is the 1C discharge cycle performance of the lithium-rich manganese-based positive electrode material in Example 3 and the charge-discharge curves of the first three weeks. After 200 cycles of charge-discharge cycles, the material prepared in this example is fully activated, and the battery capacity continues to rise to 200mAh / g or more, but after 200 charge-discharge cycles, the battery capacity drops to 160mAh / g, indicating that the cycle stability of the material is not very good, and the uniformity of the material is difficult to control.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com