Lithium-site doped and metal oxide-coated lithium ion battery positive electrode material and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, applied in the field of lithium-ion battery positive electrode materials and their preparation, can solve problems such as increased charge transfer resistance, achieve improved cycle stability, improved electrochemical performance, and the effects of inhibiting reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
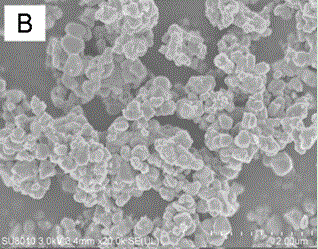
Image
Examples
Embodiment 1
[0028] Example 1 is neither manganese oxide coating nor sodium doped material Li 1.17 Ni 0.62 co 0.14 mn 0.24 o 2 The preparation of is set for comparing with the product of embodiment 3.
Embodiment 2
[0029] Example 2 is only the Li coated with manganese oxide 1.17 Ni 0.62 co 0.14 mn 0.24 o 2 0.008MnO 2 The preparation of is set for comparing with the product of embodiment 3.
Embodiment 3
[0030] Example 3 is a sodium-doped manganese oxide-coated Li 1.07 Na 0.10 Ni 0.62 co 0.14 mn 0.24 o 2 0.008MnO 2 preparation.
[0031] Example 1: Li 1.17 Ni 0.62 co 0.14 mn 0.24 o 2 preparation of
[0032] (1) Preparation of precursor
[0033] Accurately weigh the sulfates of nickel, cobalt, and manganese in a molar ratio of 0.62:0.14:0.24, add water to dissolve to form a mixed salt solution, and control the total concentration of each metal ion in the solution between 0.1mol / l and a saturated solution; Sodium carbonate that is 1.1 times the total molar number of metal ions (0.62+0.14+0.24=1) is dissolved in water to form a sodium carbonate solution with a concentration between 0.1mol / l and a saturated solution. The above two solutions were mixed and reacted completely, resulting in a suspension. Under the protection of nitrogen, the obtained suspension was continuously stirred for 24 hours, during which the pH value of the solution was precisely controlled at 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com