Preparation method of cortisone acetate
A technology of cortisone acetate and methanesulfonic acid, applied in the direction of steroids, organic chemistry, etc., can solve the problems of high cost and difficult production cost of cortisone acetate, so as to reduce production cost and improve production operation environment , the effect of improving competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
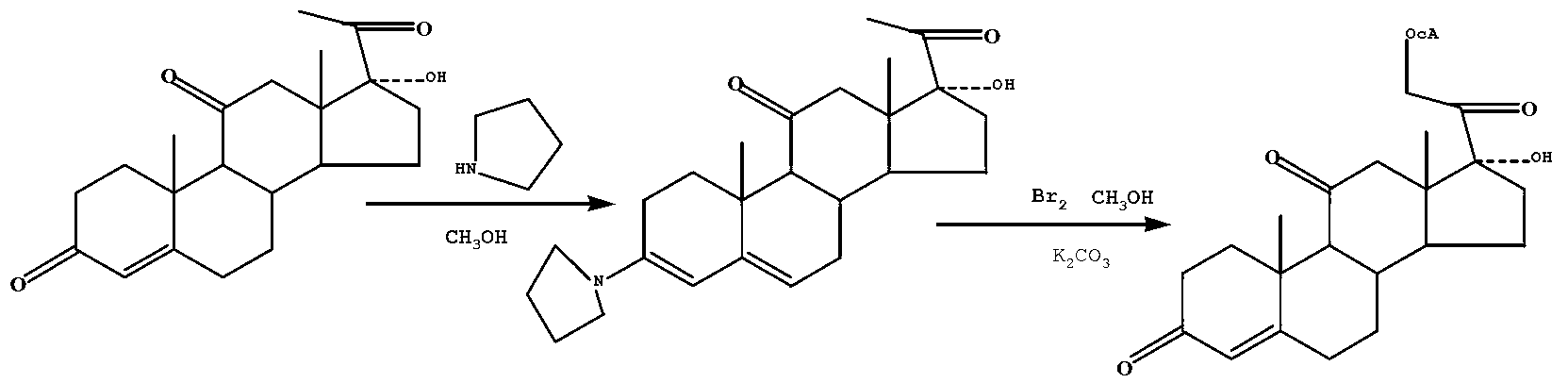
[0023] Under the protection of nitrogen flow, 115.2Kg of 17α-hydroxy-4-pregnene-3,11,20-trione was added to 270L of methanol, heated to 55°C under stirring, and then 27.95L of tetrahydropyrrole was added. After the addition is complete, keep it at 55°C for 1 hour to obtain intermediate compound A (that is, the 3-position carbonyl tetrahydropyrrole protection of 17α-hydroxy-4-pregnene-3,11,20-trione), and cool to 0 ℃, filter, drain and wash with 150L of cold methanol, then filter, drain, and the filter cake is directly used for the next reaction.
[0024] Under the protection of nitrogen flow, add the filter cake from the previous step into 1770L of methanol, add 24.5L of methanesulfonic acid under stirring, heat up to a transparent solution, then cool to room temperature, and then add 1.04L of triethyl orthoformate , add dropwise a solution made of 17.15L bromine and 217.8L methanol, dropwise for 2 hours, after the dropwise addition is complete, stir for another 15 minutes, ad...
Embodiment 2
[0026] Under the protection of nitrogen flow, 115.2Kg of 17α-hydroxy-4-pregnene-3,11,20-trione was added to 270L of methanol, heated to 55°C under stirring, then 48L of tetrahydropyrrole was added, and the After completion, keep at 55°C for 1 hour to obtain intermediate compound A (that is, the 3-position carbonyl tetrahydropyrrole protection of 17α-hydroxy-4-pregnene-3,11,20-trione), and cool to 0°C , filtered, drained and washed with 150L of cold methanol, then filtered, drained, and the filter cake was directly used for the next reaction.
[0027] Under the protection of nitrogen flow, add the filter cake from the previous step into 1770L of methanol, add 24.5L of methanesulfonic acid under stirring, heat up to a transparent solution, then cool to room temperature, and then add 2L of triethyl orthoformate, Add dropwise a solution made of 49.35L bromine and 217.8L methanol, dropwise for 2 hours, after the dropwise addition is complete, stir for another 15 minutes, add a solu...
Embodiment 3
[0029] Under the protection of nitrogen flow, 115.2Kg of 17α-hydroxy-4-pregnene-3,11,20-trione was added to 270L of methanol, heated to 55°C under stirring, and then 55.9L of tetrahydropyrrole was added. After the addition is complete, keep it at 55°C for 1 hour to obtain intermediate compound A (that is, the 3-position carbonyl tetrahydropyrrole protection of 17α-hydroxy-4-pregnene-3,11,20-trione), and cool to 0 ℃, filter, drain and wash with 150L of cold methanol, then filter, drain, and the filter cake is directly used for the next reaction.
[0030] Under the protection of nitrogen flow, add the filter cake from the previous step into 1770L of methanol, add 24.5L of methanesulfonic acid under stirring, heat up to a transparent solution, then cool to room temperature, and then add 3.46L of triethyl orthoformate , add dropwise a solution made of 51.46L bromine and 217.8L methanol, dropwise for 2 hours, after the dropwise addition is complete, stir for another 15 minutes, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com