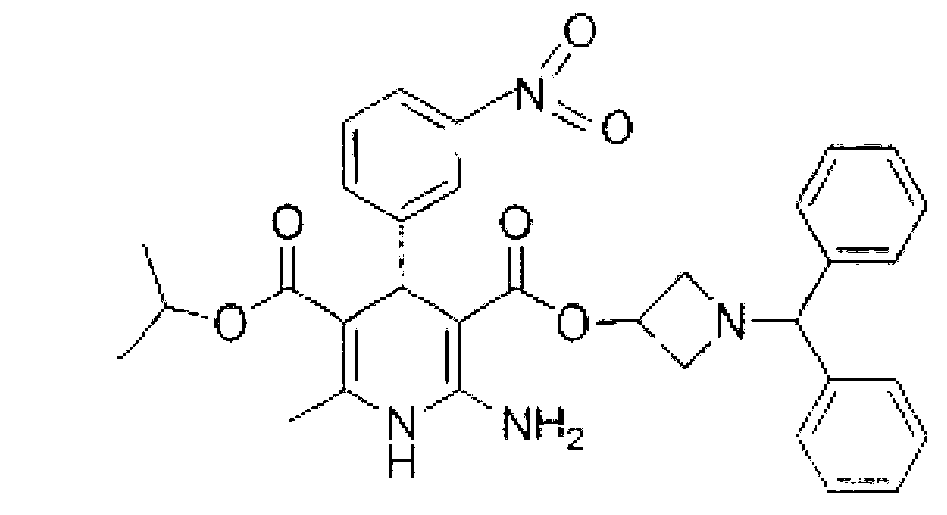
Azelnidipine tablets and preparation method thereof
A technology of Azelnidipine and Dipine Tablets, which is applied in the field of Azelnidipine Tablets and its preparation, can solve the problems of difficulty in guaranteeing the stability of Azelnidipine, partial acidity of β-cyclodextrin, etc., and reduce the types of excipients and the dissolution rate Improvement and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
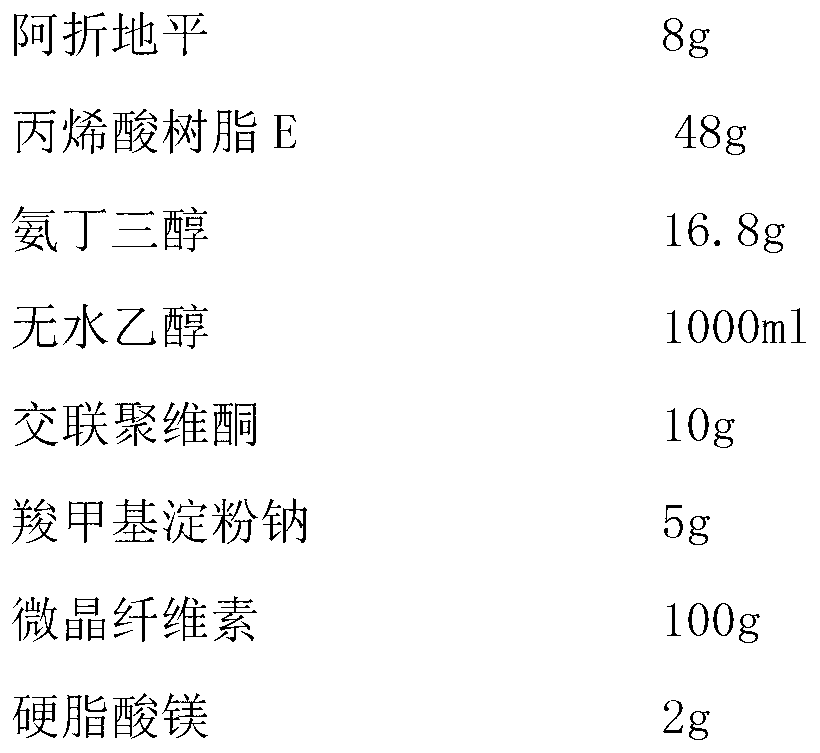
[0023]
[0024] Preparation Process:
[0025] (1) Dissolve azedipine, acrylic resin E and tromethamine in absolute ethanol, dry under reduced pressure, pass the dry matter through a 100-mesh sieve to obtain a solid dispersion, and set aside;
[0026] (2) Pass sodium carboxymethyl starch, microcrystalline cellulose and lactose through a 100-mesh sieve, and set aside;
[0027] (3) Prescription quantity Weigh the sieved solid dispersion, sodium carboxymethyl starch, microcrystalline cellulose, lactose, and magnesium stearate, mix well, and press into tablets.
Embodiment 2
[0029]
[0030] Preparation Process:
[0031] (1) Dissolve azedipine, acrylic resin E, and tromethamine in absolute ethanol, dry under reduced pressure, pass the dry matter through a 100-mesh sieve to obtain a solid dispersion, and set aside;
[0032] (2) Pass sodium carboxymethyl starch, crospovidone, and microcrystalline cellulose through a 100-mesh sieve, and set aside;
[0033] (3) Prescription quantity Weigh the sieved solid dispersion, sodium carboxymethyl starch, crospovidone, microcrystalline cellulose, and magnesium stearate, mix well, and press into tablets.
Embodiment 3
[0035]
[0036] Preparation Process:
[0037] (1) Dissolve azedipine, acrylic resin E and tromethamine in absolute ethanol, dry under reduced pressure, pass the dry matter through a 100-mesh sieve to obtain a solid dispersion, and set aside;
[0038] (2) Pass sodium carboxymethyl starch and pregelatinized starch through a 100-mesh sieve, and set aside;
[0039] (3) Prescription quantity Weigh the sieved solid dispersion, sodium carboxymethyl starch, pregelatinized starch, and sodium stearate fumarate, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com