Friedelane triterpenoid and preparation method as well as application thereof
A technology of triterpenoids and compounds of suberane type, which is applied in the fields of steroids, organic chemistry, and pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
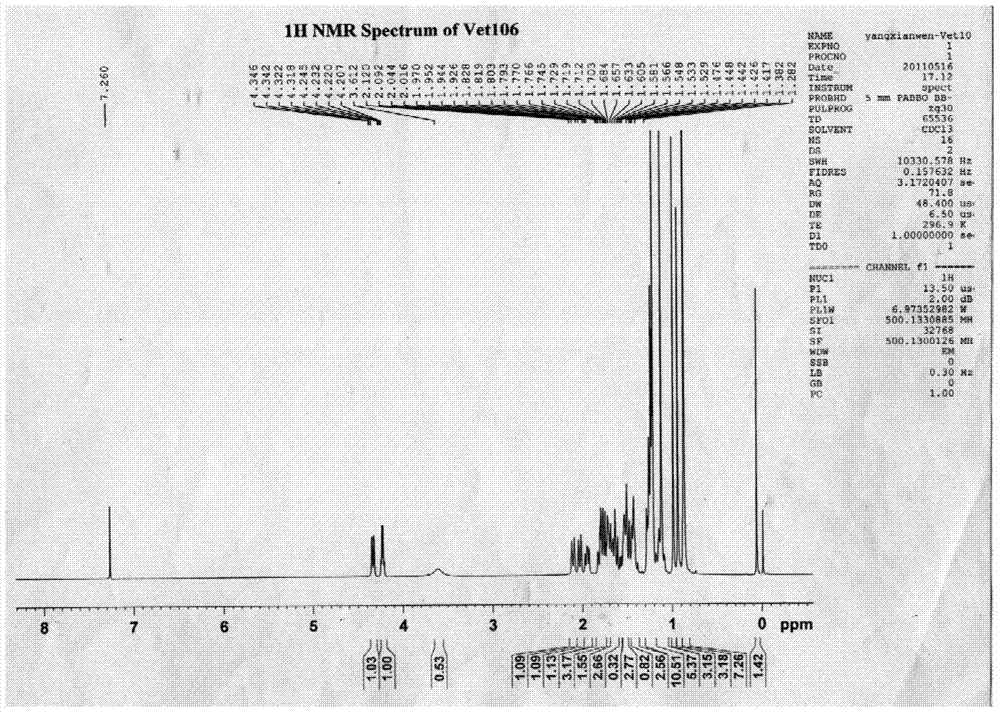
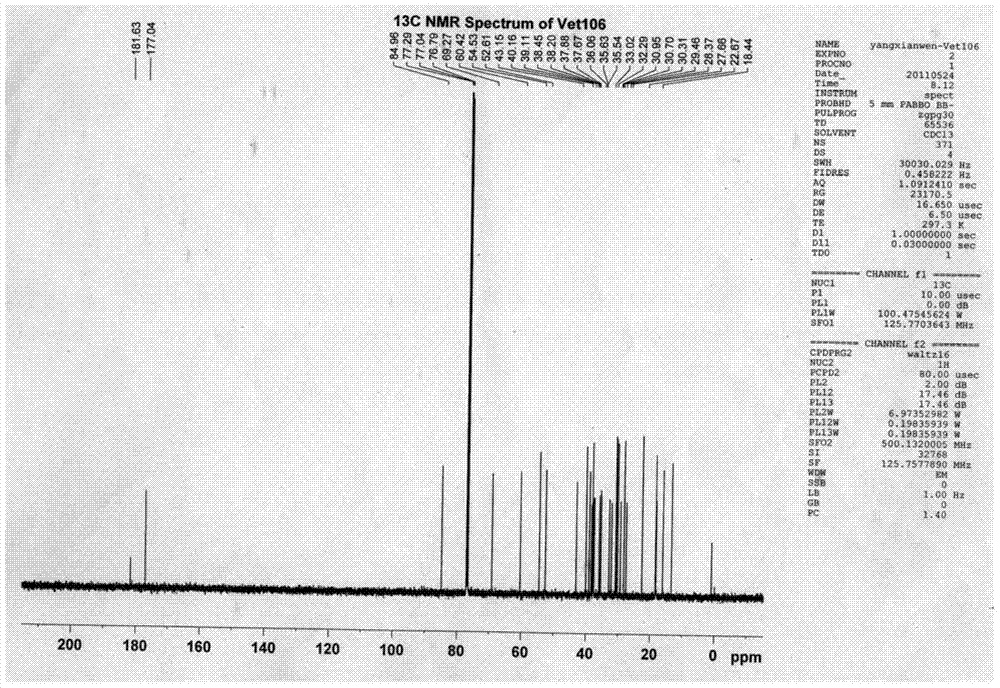
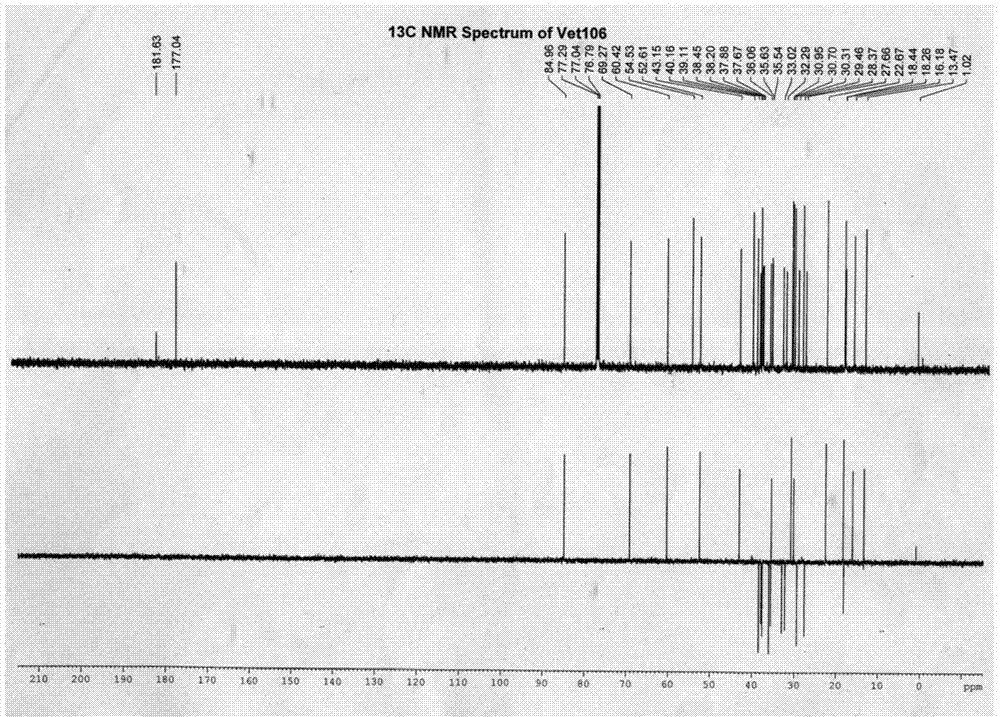
[0055] 1. Preparation of compounds
[0056] (1) Take 10kg of dried Viola vine herb, reflux with 120L, 100L and 80L of 95% ethanol for 2 hours, combine the ethanol extract, recover the ethanol and concentrate it under reduced pressure to nothing. Alcoholic taste, get the total extract;
[0057] (2) Dissolve the total extract in water, then extract 3 times with 1.4L petroleum ether, 3L ethyl acetate and 3L water-saturated n-butanol successively, take the n-butanol extract, concentrate under reduced pressure to remove the solvent, and obtain Butanol extract 168g;
[0058] (3) Dissolve 84g of n-butanol extract in 600ml of water, pass through a D-101 macroporous resin column, and then use water, ethanol with a concentration of 10%, ethanol with a concentration of 30%, ethanol with a concentration of 60%, and ethanol with a concentration of Elute with 90% ethanol and 95% ethanol; collect the 95% ethanol eluate through a 200-300-mesh silica gel column, use petroleum ether-ethyl ace...
example 2
[0067] Example 2: (injection)
[0068] Take 1000 mg of the compound obtained by the method described in Example 1 above, add 1000 ml of water for injection, adjust the pH value to 7-7.5 with sodium carbonate, stir to dissolve, sterilize and filter, potting, and circulate steam at 100 ° C for 15 minutes Sterilize and make each 2mg / 2ml injection for injection.
example 3
[0069] Example 3: (capsules)
[0070] Take the compound 5000mg obtained by the method described in the above-mentioned Example 1 and fully mix with auxiliary materials such as 4000mg microcrystalline cellulose, 500mg sodium carboxymethyl starch, 400mg sodium lauryl sulfate, and carry out dry granulation by roller compaction, and then mix with An appropriate amount of magnesium stearate is mixed, filled into 3# hollow capsules, and made into capsules with a specification of 100 mg / grain for oral use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com