Rhodamine 6G-based Cu<2+> fluorescence probe molecule and preparation method thereof
A technology of fluorescent probe and rhodamine hydrazide, which is applied in the field of metal ion analysis and detection, achieves the effects of simple preparation method, strong selectivity and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Fluorescent probe molecule of the present invention L Synthetic method:
[0030] Step 1: Rhodamine 6G hydrazide is synthesized according to the method of document J. AM. CHEM. SOC. 2005, 127, 16760-16761.
[0031]Step 2: Add rhodamine hydrazide (214.0mg, 0.5mmol) and methyl 3-formyl-2-hydroxybenzoate (90.1mg, 0.5mmol) into 10ml methanol solution, mix well, then add a few drops of glacial acetic acid . Stir and reflux at 68°C for 2 hours, cool to room temperature, a light pink precipitate is formed, filter, wash the precipitate several times with a 1:1 mixture of methanol and ether, and then dry it in vacuum. The obtained solid was 253.8 mg, and the yield was 86%.
[0032]
Embodiment 2
[0033] Example 2 Fluorescent probe molecule of the present invention L in Application experiments in the detection of copper ions:
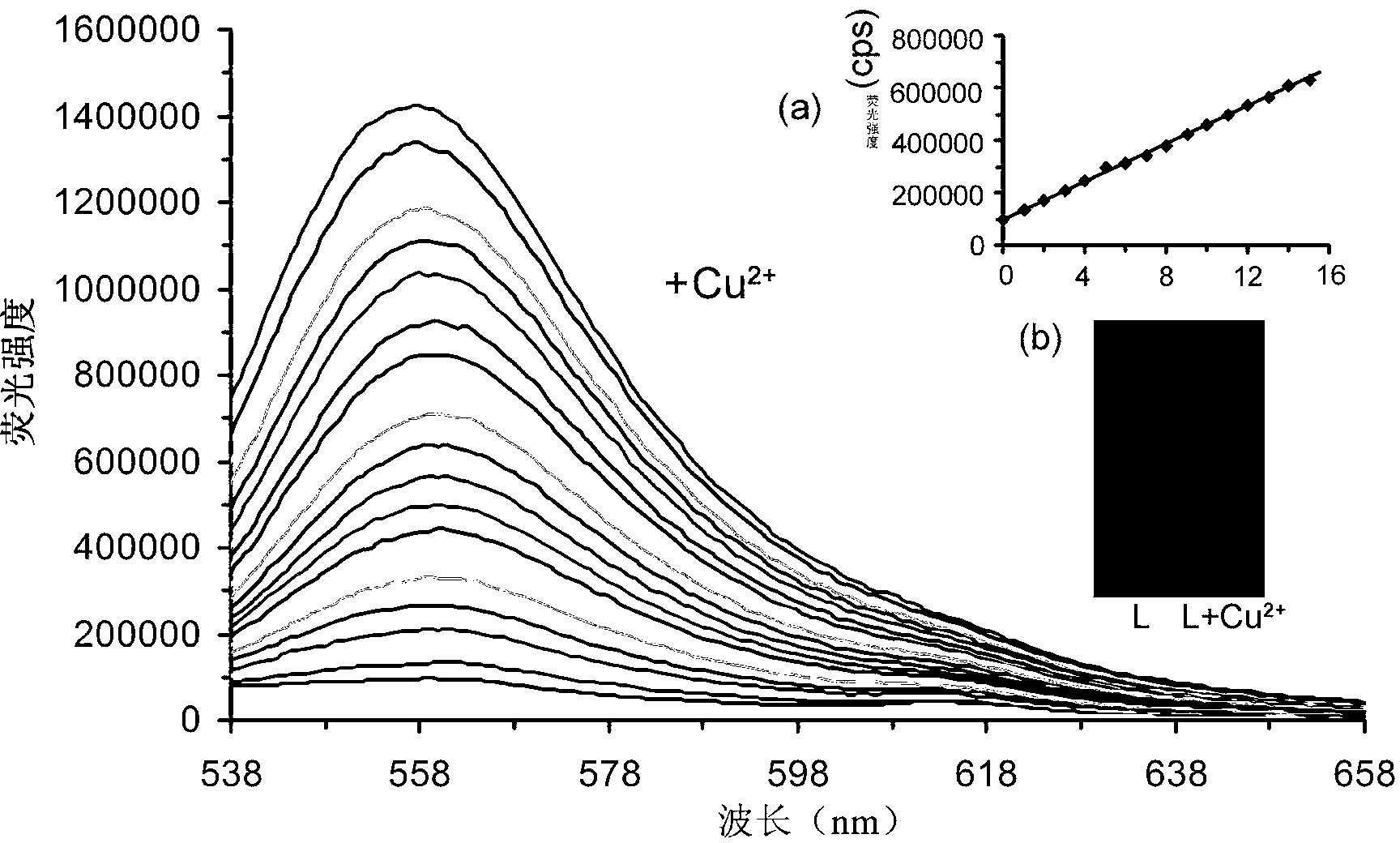
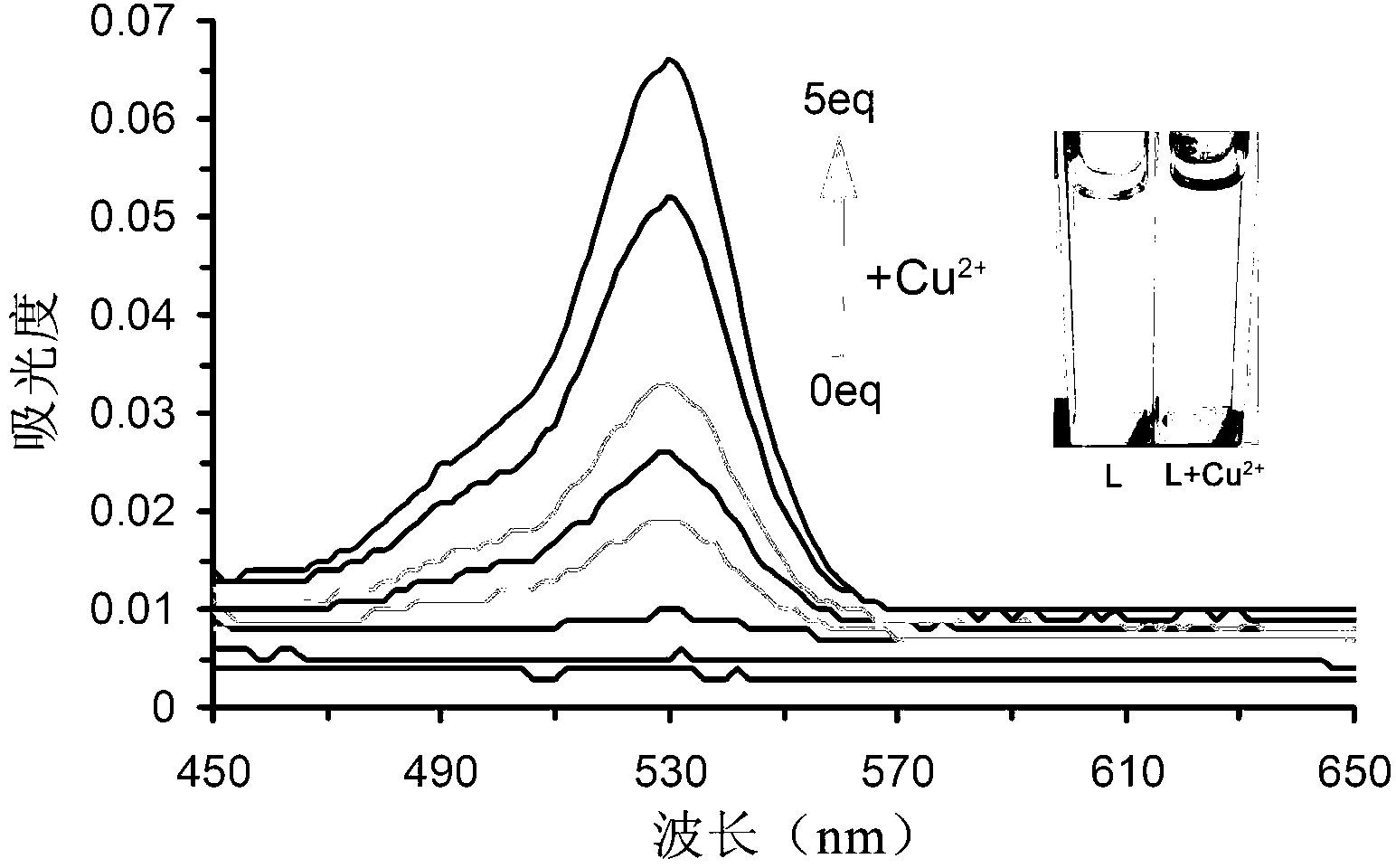
[0034] Prepare 5mM aqueous solutions of various metal salts with deionized water. Weigh 2.95mg fluorescent probe molecule of the present invention L, It was dissolved in 25 ml of N,N-2-methylformamide solution (DMF) to prepare a 0.2 mM solution. Add 3ml of N,N-2-methylformamide to the cuvette, and then add 75 μL of 0.2mM fluorescent probe molecules of the present invention configured above L solution, which was diluted to 5 μM. After mixing evenly, use 521nm as the excitation wavelength to measure the fluorescence value in the range of 538nm-700nm.
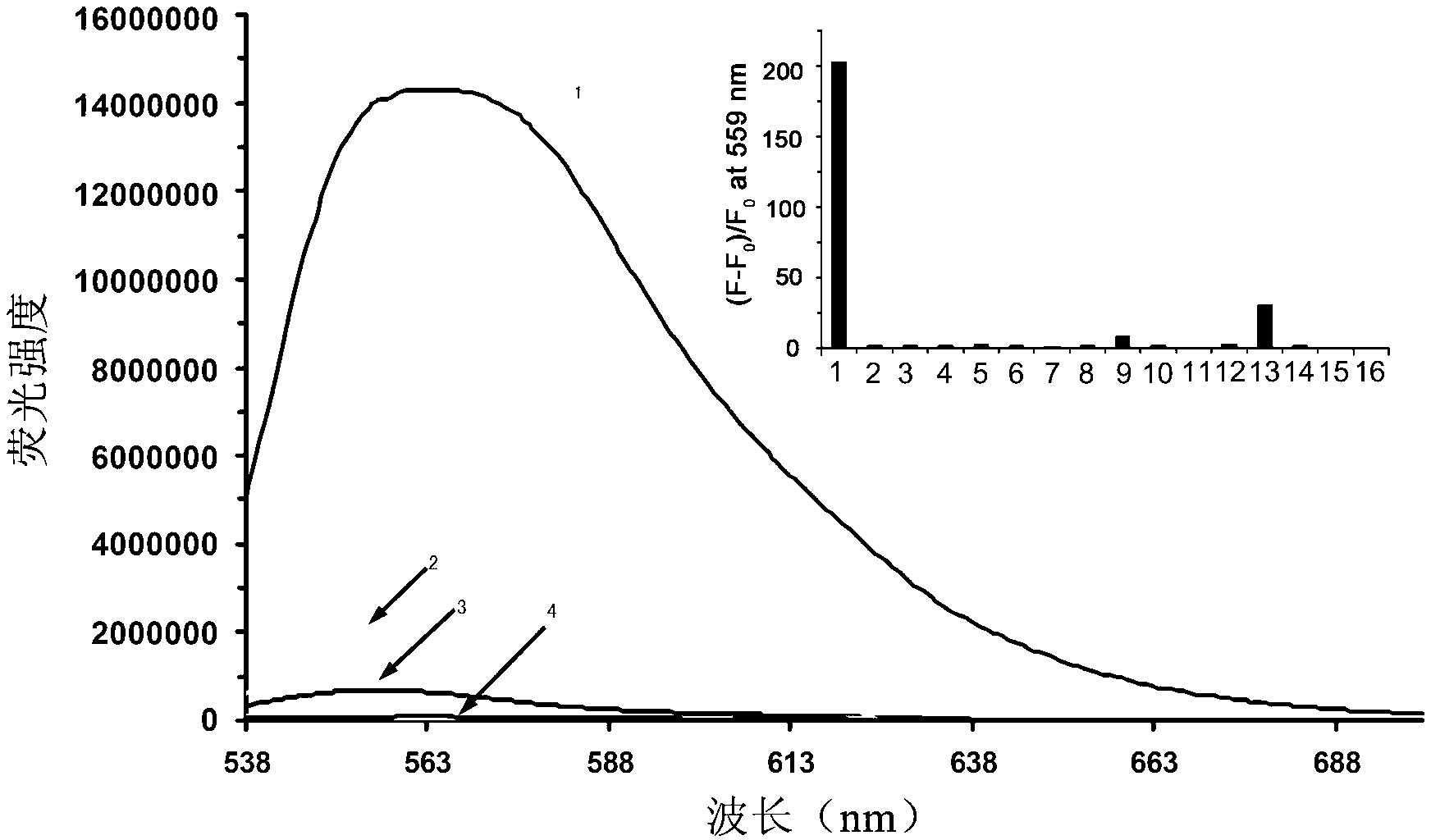
[0035] Fluorescent probe molecule of the present invention L Selectivity: 5 μM fluorescent probe molecules of the present invention L There is only weak fluorescence at 559nm, add 10 equivalents of various metal ions to the system, as attached figure 1 As shown, only the addition of copper io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com