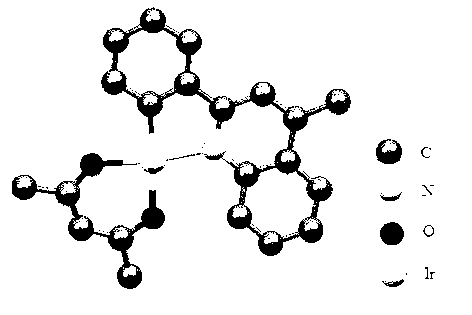
Polysubstituted phenylquinoline platinum (II) complex as well as preparation method and application thereof
A phenylquinoline platinum, multi-substitution technology, applied in the field of multi-substituted phenylquinoline platinum complexes, can solve the problem of reducing the quantum efficiency of luminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of complex [2(4FPh)4Me]Pt(acac)
[0040]
[0041] (1) 4-Methylquinoline-2(1 H )-Ketone synthesis
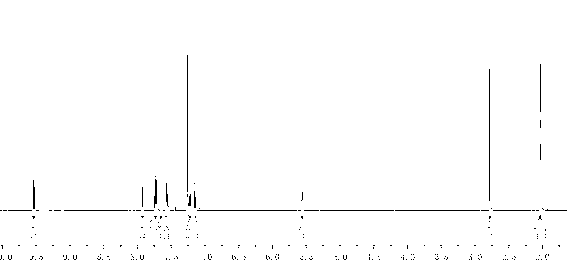
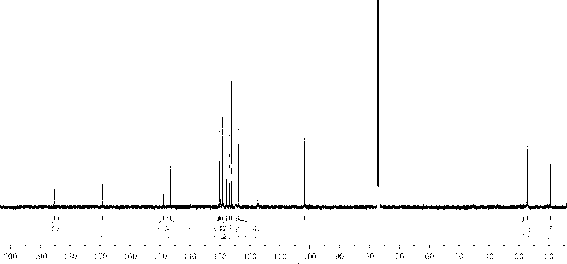
[0042] Put 40mL concentrated sulfuric acid into a three-necked flask with a stirrer, gradually add 17.7g N-acetoacetanilide at room temperature, add 10mL concentrated sulfuric acid after the addition, heat up to 80℃ and react for 10min, carefully pour into 400mL ice water, and precipitate A large amount of white precipitate, filtered under reduced pressure, washed with water until neutral, dried under vacuum at 30°C to obtain 4-methylquinoline-2 (1 H )-Ketone white solid, colorless rod-shaped crystals are precipitated out by ethanol recrystallization, the yield is 87%. 1 H NMR (600MHz, CDCl 3 ) δ(ppm): 11.95(br; 1H); 7.70(dd; J =1.20Hz; J =7.20Hz; 1H); 7.52(dt; J =1.20Hz; J =7.20Hz; 1H); 7.42(dd; J =1.20Hz; J =7.20Hz; 1H); 7.26(dt; J =1.20Hz; J =7.20Hz; 1H); 6.61(q; J =1.20Hz; 1H); 2.53(d; J =1.20Hz; 3H).
[0043] (2) Synthesis of 2-chloro-4-methylquinoline
...
Embodiment 2
[0051] Example 2: Synthesis of complex (2Ph4Me)Pt(acac)
[0052]
[0053] (1) Synthesis of 2-phenyl-4-methylquinoline
[0054] Take 3.0 g of 2-chloro-4-methylquinoline synthesized in Example 1, 2.06 g of phenylboronic acid, and Pd(PPh 3 ) 4 640mg, 20mL of saturated sodium carbonate aqueous solution, 10mL of anhydrous methanol, 40mL of toluene, were added to a three-necked flask with a stirrer, nitrogen gas, reflux reaction for 14h, discard the water layer, and extract the toluene layer with an aqueous sulfuric acid solution of appropriate concentration. After the extraction is complete, the acidic aqueous layer is combined and filtered to obtain a colorless and clear aqueous layer. The acidic aqueous phase is adjusted to weakly alkaline with potassium hydroxide solution of appropriate concentration, and a large amount of white solid is precipitated. The target product is obtained by static filtration or extraction with ethyl acetate. After vacuum drying at 35°C, 3.55 g of white cr...
Embodiment 3
[0067] Example 3: Synthesis of complex [2(224FPh)4Me]Pt(acac)
[0068]
[0069] (1) Synthesis of 2-(2,4-difluorophenyl)-4-methylquinoline
[0070] Take 3.0 g of 2-chloro-4-methylquinoline synthesized in Example 1, 2.67 g of 2,4-difluorophenylboronic acid, and Pd(PPh 3 ) 4 640mg, 20mL of saturated sodium carbonate aqueous solution, 10mL of anhydrous methanol, 40mL of toluene, were added to a three-necked flask with a stirrer, nitrogen gas, reflux reaction for 14h, after the reaction, the water layer was discarded, and the toluene was extracted with an aqueous sulfuric acid solution of appropriate concentration After multiple extractions, combine the acidic water layers and filter to obtain a colorless and clear water layer. Adjust the acidic water phase to weakly alkaline with a potassium hydroxide solution of appropriate concentration. A large amount of white solids precipitate out, static filtration or extraction with ethyl acetate The target product was obtained, which was vacuu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com