Continuous indigo blue electrochemistry reduction dyeing process
An electrochemical and indigo technology, applied in the field of vat dyeing technology, can solve the problems of affecting dyeing quality, low current density, high production cost, etc., achieve high reaction efficiency, reduce medium loss, and ensure quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
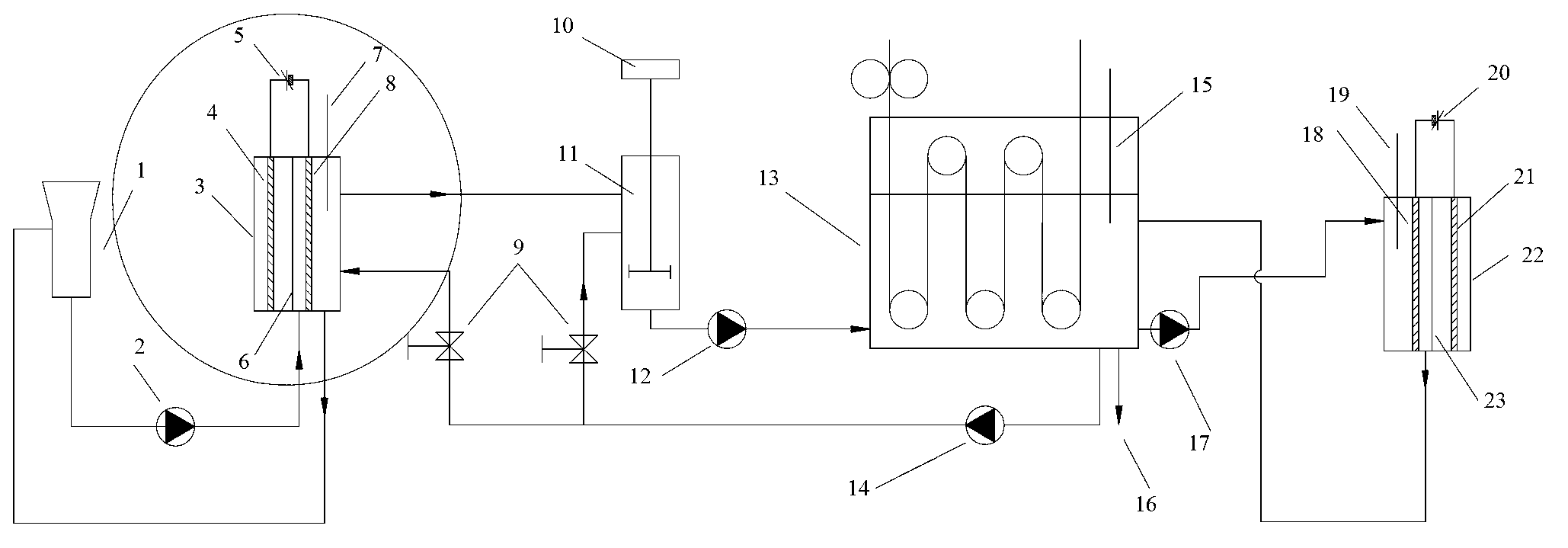
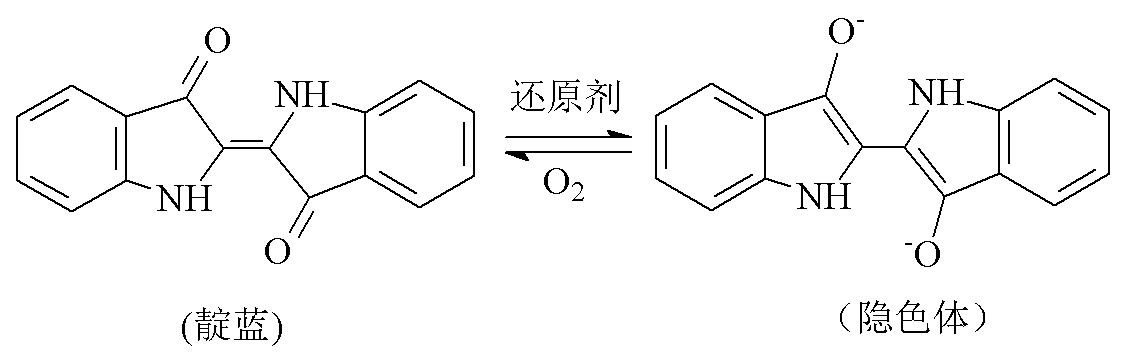
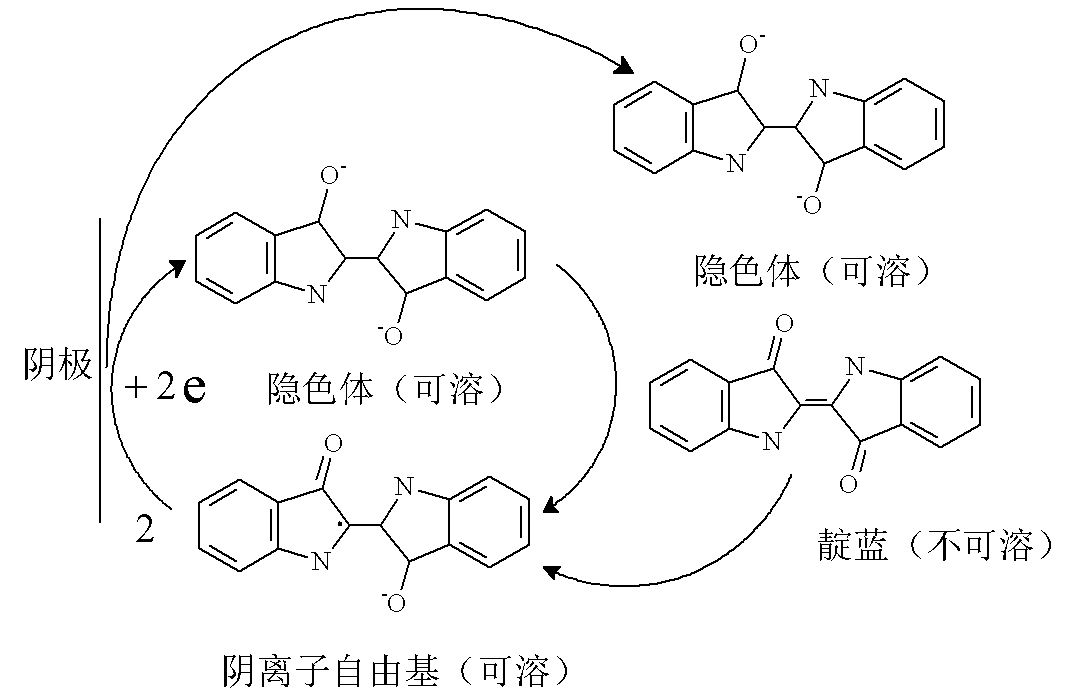
[0033] In 0.2 mol / L NaOH aqueous solution, add indigo (0.02 mol / L), Fe 2 (SO 4 ) 3 (0.0005 mol / L), TEA (0.012 mol / L) and other substances are configured as the initial catholyte, and the electrolyte is passed into the electrochemical reactor (3) for electrolytic reduction. The temperature of the electrolytic reaction is 40°C, and the current density of the single-piece stainless steel cathode mesh is 0.2 A / dm 2 ; In the electrochemical reactor (3), the cathode is a piece of stainless steel mesh, the Nafion324 cationic membrane is a diaphragm, the anode is a ruthenium iridium electrode (purchased from Hangzhou Sailong Chemical Co., Ltd.), and the anolyte is 1 mol / L H 2 SO 4 . After 80% of the indigo is reduced to the leucosome, start to continuously output the electrolyte solution to the leucosome dilution tank (11) for dilution, and at the same time continuously input the dyed solution from the feeding tank (1) or the electrochemical reactor (3) at the same flow rate The...
Embodiment 2
[0035] In 0.2 mol / L NaOH aqueous solution, add indigo (2.0 mol / L), Fe 2 (SO 4 ) 3 (0.01 mol / L), TEA (0.15 mol / L) and other substances are configured as the initial catholyte, and the electrolyte is passed into the electrochemical reactor (3) for electrolytic reduction. The temperature of the electrolytic reaction is 60°C, and the current density of the single-piece stainless steel cathode mesh is 5 A / dm 2 ; In the electrochemical reactor (3), the cathode is 5 stainless steel meshes separated from each other, the Nafion117 cationic membrane is the diaphragm, the anode is the ruthenium iridium electrode, and the anolyte is 0.5 mol / L H 2 SO 4 . After 98% of the indigo is reduced to the leucosome, start to continuously output the electrolyte solution to the leucosome dilution tank (11) for dilution, and at the same time continuously input the dyed solution from the feeding tank (1) or the electrochemical reactor (3) at the same flow rate After filtering the dyeing solution, ...
Embodiment 3
[0038] At 1.0 mol / L Na 2 CO 3 In the aqueous solution, add indigo (1.0 mol / L), Fe 2 (SO 4 ) 3 (0.02 mol / L), TEA (0.3 mol / L) and other substances are configured as an electrolyte, and the electrolyte is passed into the electrochemical reactor (3) for electrolytic reduction. The temperature of the electrolytic reaction is 20°C, and the current density of the single-piece stainless steel cathode mesh is 2 A / dm 2 ; In the electrochemical reactor (3), the cathode is 10 stainless steel meshes separated from each other, the Nafion117 cationic membrane is a diaphragm, the anode is a ruthenium iridium electrode, and the anolyte is 0.5 mol / L H 2 SO 4 . After 95% of the indigo is reduced to the leucosome, start to continuously output the electrolyte solution to the leucosome dilution tank (11) for dilution, and at the same time continuously input the dyed solution from the feeding tank (1) or the electrochemical reactor (3) at the same flow rate After filtering the dyeing solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com