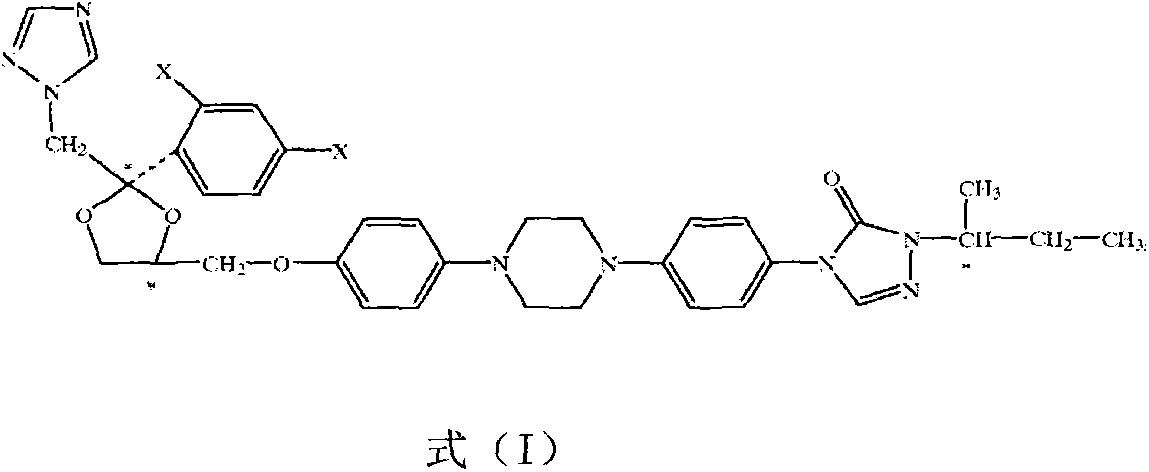
Itraconazole isomer and medical application thereof
A technology of itraconazole and isomers, which is applied in the field of medical application of 2’S itraconazole isomer combinations, can solve the problem that itraconazole liver cell damage cannot be confirmed by the liver cytotoxicity model
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
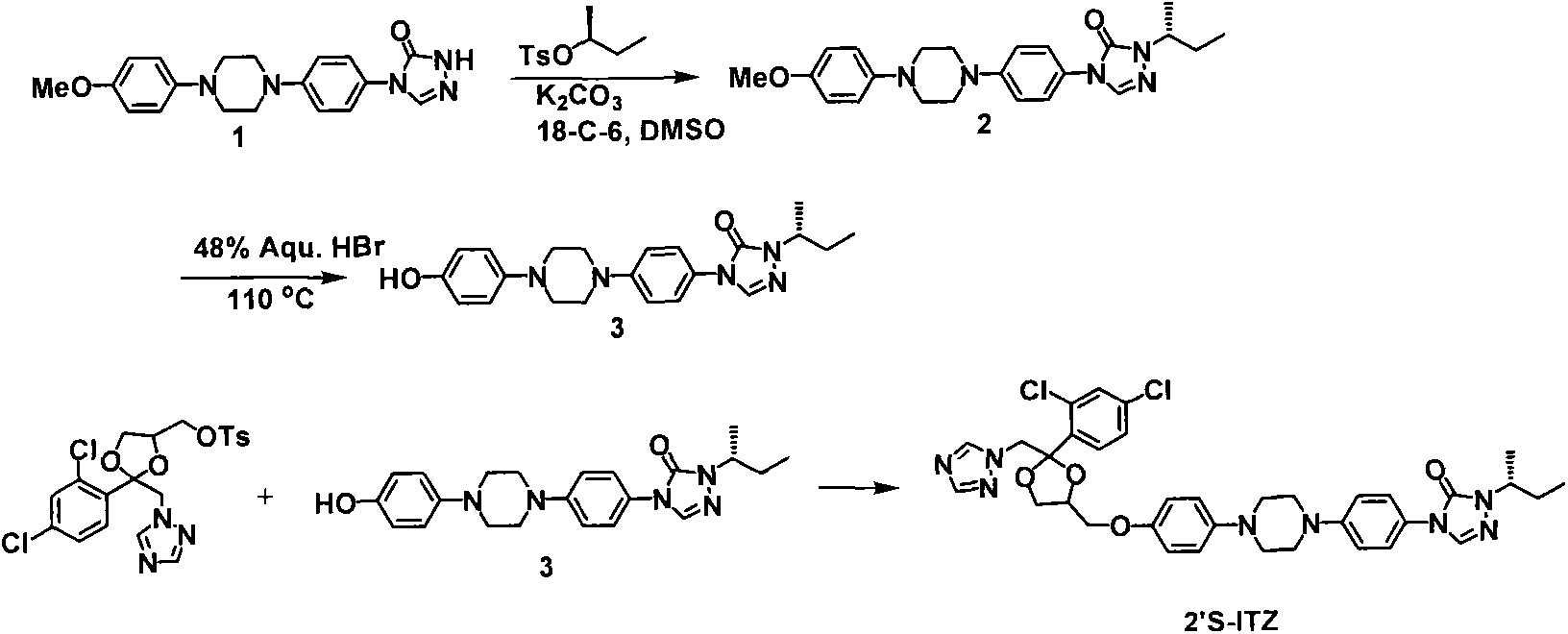
[0031] Embodiment 1: the synthesis of itraconazole 2'S isomer
[0032] step 1:
[0033]
[0034] K 2 CO 3 (0.26g, 1.92mmol), crown ether 18-C-6 (0.34g, 1.28mmol) and tosylate (0.44g, 1.92mmol). The resulting mixture was stirred overnight at room temperature, diluted with water and washed with CH 2 Cl 2 of extraction. The organic layers were combined and dried (Na 2 SO 4 ), filtered, concentrated to obtain crude product, and purified: column chromatography (dichloromethane: acetone = 10: 1) to obtain white solid 2 (0.27g, 51%).
[0035] Step 2:
[0036]
[0037] A solution of triazolone 2 (0.50 g, 1.2 mmol) was added to HBr (48%, 5 mL). The reaction mixture was heated to 110°C and refluxed overnight. The reaction mixture was cooled to room temperature and a pink solid precipitated. The solid was collected by filtration, dissolved in methanol-water (1:1, 40 mL) and saturated NaHCO 3 aqueous solution (20 mL) and extracted with chloroform (3 x 20 mL). The organi...
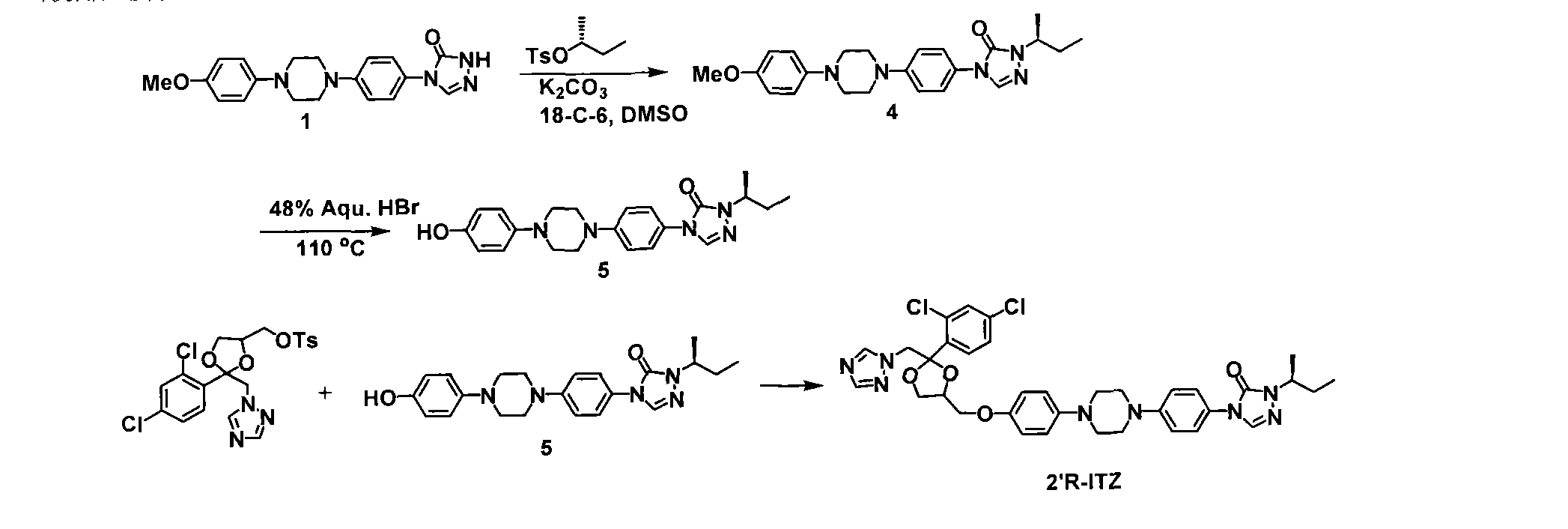
Embodiment 2
[0042] Example 2: Synthesis of 2'R isomers
[0043]
[0044] K 2 CO 3 (0.26g, 1.92mmol), crown ether 18-C-6 (0.17g, 0.6mmol) and tosylate (0.22g, 1.0mmol). The resulting mixture was stirred overnight at room temperature, diluted with water and washed with CH 2 Cl 2 of extraction. The organic layers were combined and dried (Na 2 SO 4 ), filtered, concentrated to obtain the crude product, and purified: column chromatography (dichloromethane: acetone = 10: 1) to obtain white solid 4 (0.12 g, 51%).
[0045]
[0046] A solution of triazolone 2 (0.50 g, 1.2 mmol) was added to HBr (48%, 5 mL). The reaction mixture was heated to 110°C and refluxed overnight. The reaction mixture was cooled to room temperature and a pink solid precipitated. The solid was collected by filtration, dissolved in methanol-water (1:1, 40 mL) and saturated NaHCO 3 aqueous solution (20 mL) and extracted with chloroform (3 x 20 mL). The organic layer was washed with brine and washed with MgSO ...
Embodiment 3
[0050] Example 3: Antifungal Activity
[0051] Take a sterile 96-well plate, and add 100ul of RPMI1640 culture solution in the first space of each row as a blank control, add 100ul of freshly prepared bacterial solution in each of the 3-12 wells, and add 180ul of the bacterial solution and itraconazole or Isomer solution 20ul, 2-11 wells were diluted 10 times, the drug concentration in each well was 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125mg / L. The content of dimethyl sulfoxide in each well was lower than 1%. Candida, Cryptococcus neoformans, and filamentous bacteria were cultured at 35°C for 24 hours, 72 hours, and 7 days respectively, and the dimethyl sulfoxide content in each well was measured at 630nm with an enzyme label analyzer. OD value, compared with the positive control well, the lowest concentration at which the OD value decreases by more than 80% is MIC80 (representing that 80% of the fungal growth is inhibited).
[0052] Antibacterial test results in vitro (mg / L)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com