Replicon DNA (deoxyribonucleic acid) vaccine vector, and construction method and application thereof
A DNA vaccine and replicon technology, applied in the direction of recombinant DNA technology, the use of vectors to introduce foreign genetic material, pharmaceutical formulations, etc., can solve the problems that are not suitable for the development of clinical biotherapeutic drugs, etc., and achieve rapid measurement, broad application prospects, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1, Construction of Replicon DNA Vaccine Vector pSVK
[0061] The construction method of the replicon DNA vaccine vector pSVK of the present invention comprises the following steps:
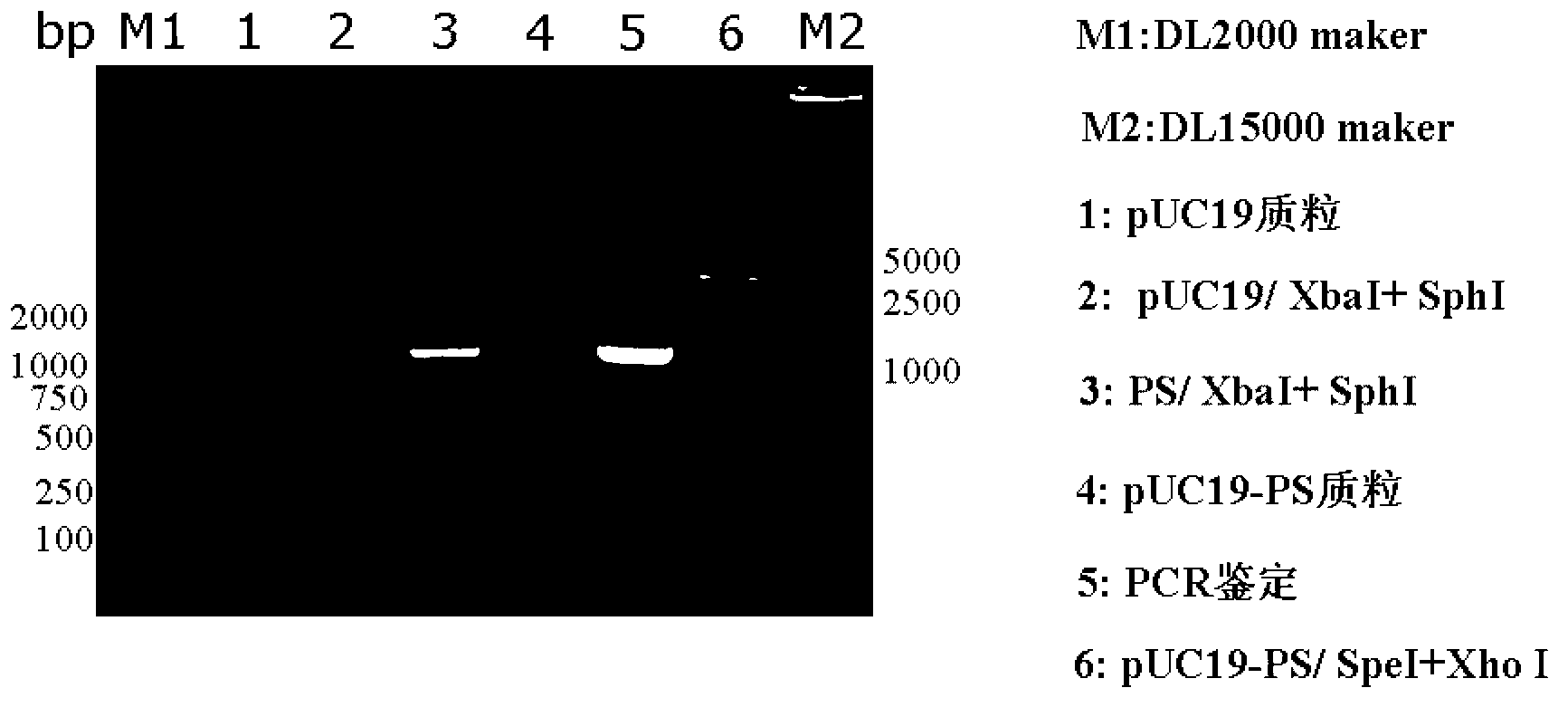
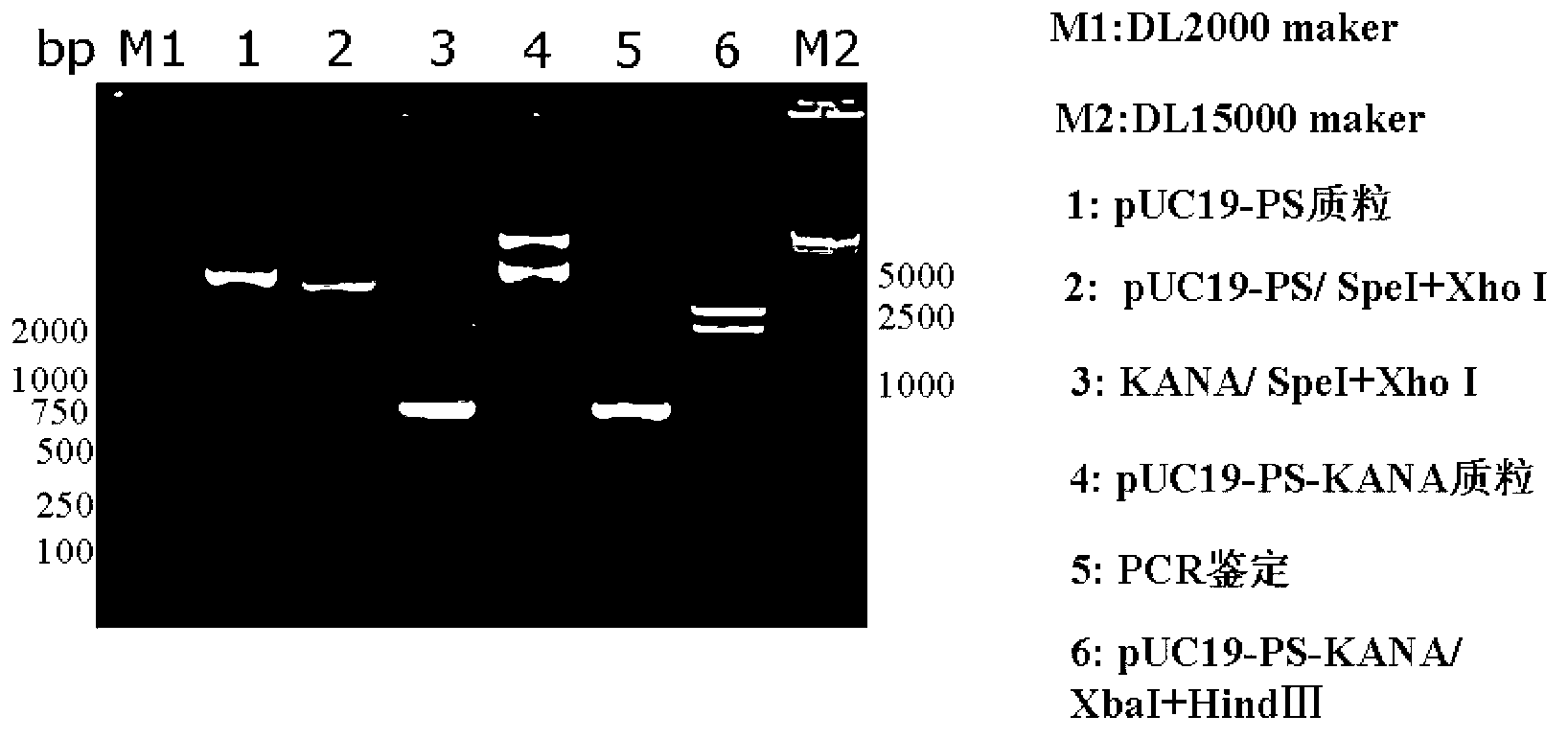
[0062] 1. Construction of recombinant vector pUC19-PS-KANA carrying PS and KANA fusion gene (PS-KANA)
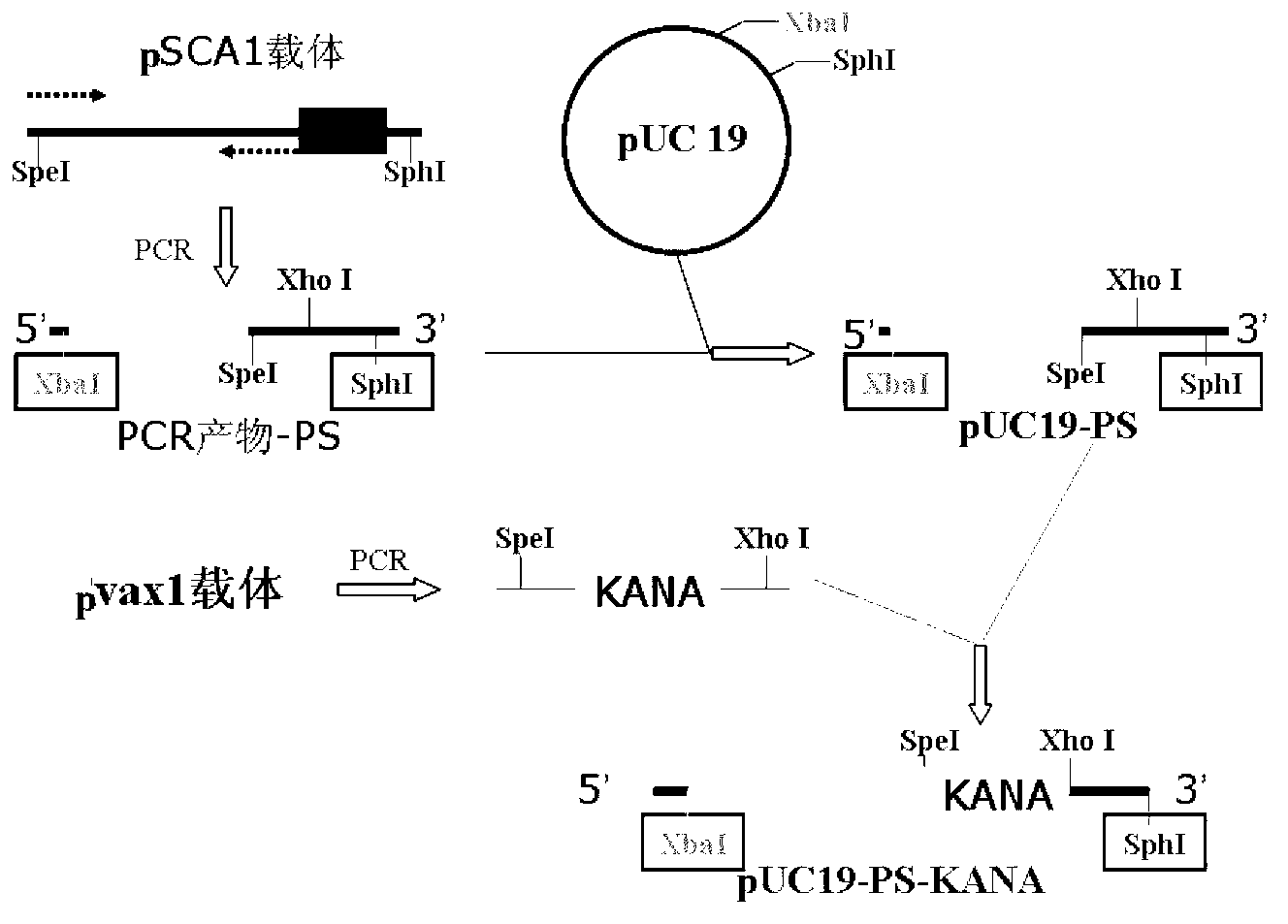
[0063] Such as figure 1 As shown, the construction method of the recombinant vector pUC19-PS-KANA is as follows:
[0064] 1) Amplify the PS gene: use the replicon DNA vaccine vector pSCA1 containing the ampicillin resistance gene (for the construction method, refer to the literature Yu Y Z, Sun Z W, Yu W Y. Chinese Journal of Biotechnology, 2005, 21(5): 33- 38) as a template, under the guidance of primers PSCA-F (5'-CCGTCTAGAGATCATAATCAGCCAT-3') and PSCA-R (5'-CCGGCATGCCTCGAGACTAGTCTGTCAGACCAAG-3'), PCR amplifies the flanking sequence of the ampicillin resistance gene, the The side sequence at the 5' end contains a restriction endonuclease Xba I recognition site, and the side s...
Embodiment 2
[0069] Example 2, Construction and in vitro expression of replicable luciferase expression plasmid pSVK-luc
[0070] 1. Construction of replicable luciferase expression plasmid pSVK-luc
[0071] The firefly luciferase (luciferase) gene (GenBank No.: JN244035.1) was cloned into the replicon DNA vaccine vector pSVK modified for resistance in Example 1 to obtain the replicable luciferase expression plasmid pSVK-luc, specifically constructed Methods as below:
[0072] Using firefly luciferase reporter vector pGL-3-CMV (Zhang Liang, Li Xiaoxiao, Han Gang, Dong Jinkai, Yan Jinqi, Xiao Yi, Yu Jiyun. Experimental study on the effect of delivering plasmid DNA in different ways to induce expression in vivo. Journal of Military Medical Training College. 20101;31 (1):48-49) was used as a template, and the firefly luciferase gene luc was amplified by PCR under the guidance of primers Luc-F (5'-ATAGGATCCGCCACCATGGAAGACGC-3) and Luc-R (5'-ATTCCCGGGTTACACGGCGATCTTTC-3'); The PCR reaction sy...
Embodiment 3
[0076] Example 3. In vivo expression function verification of pSVK-luc and study on electroporation delivery conditions
[0077] Replicon DNA vaccine is a new type of vaccine with great development potential, which is extremely beneficial to the research of new therapeutic vaccines for major chronic diseases, but the research on how to efficiently deliver new plasmid DNA vaccines in vivo is still very limited. The recently developed high-efficiency technology uses high-intensity electric fields to instantly increase the permeability of cell membranes and introduce nucleic acids, proteins, and other foreign molecules into cells. In order to further study the specific conditions of in vivo electroporation delivery of the replicon DNA vaccine and the dynamic expression of the vaccine in vivo, the present invention simulates the immunization process of the replicon DNA vaccine through bioluminescent technology in vivo, and studies the possible delivery of the replicon DNA vaccine. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com