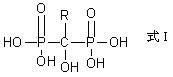
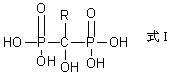
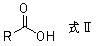Preparation method for diphosphonic acid compound
A compound and bisphosphonic acid technology, applied in the field of preparation of bisphosphonic acid compounds, can solve the problems of complex reaction conditions, toxic solvents, corrosiveness and the like, and achieve the effects of simple reaction conditions, convenient industrialization and non-corrosiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Take n-hexane (200 mL) and water (1.1 mL), put them in a double-neck flask, -20°C, under stirring, add PCl dropwise 3 (20.0 mmol), stirred for 0.5 h. At -20°C, add 2-(1 H -1-imidazolyl)-acetic acid (20.0 mmol). to be 2-(1 H -1-imidazolyl)-acetic acid is completely dissolved, at -20 ℃, drop into PCl 3 (30.0 mmol), warmed up to 50°C, and stirred for 8 h. Add 0.1mol / L hydrochloric acid (200 mL), and react at 80°C for 8 h. After cooling to room temperature, the organic layer was decanted. After the aqueous layer was concentrated under reduced pressure, it was dripped into 12.6 mL of acetone at -20°C, left to stand for crystallization at -20°C for 1 h, and filtered with suction. The filter cake was dried at 20°C for 5 h to obtain the target compound—zoledronic acid monohydrate (16.2 mmol, yield 81%, purity 99.5% by HPLC normalization method).
Embodiment 2
[0044] Take n-decane (50 mL) and water (3.0 mL), put them in a double-neck flask, 0 ° C, under stirring, add PCl dropwise 3 (50.0 mmol), stirred for 2 h. At 25°C, add 2-(1 H -1-imidazolyl)-acetic acid (20.0 mmol). to be 2-(1 H -1-imidazolyl)-acetic acid is completely dissolved, at 0 ℃, drop into PCl 3 (80.0 mmol), heated to 70°C and stirred for 18 h. Concentrated hydrochloric acid (10 mL) was added and reacted at 95°C for 18 h. After cooling to room temperature, the organic layer was decanted. After the aqueous layer was concentrated under reduced pressure, it was dropped into 60 mL of methanol at 0°C, and allowed to stand for crystallization at -10°C for 8 hours, and then suction filtered. The filter cake was dried at 60°C for 5 h to obtain the target compound—zoledronic acid monohydrate (18.2 mmol, yield 91%, purity 99.8% by HPLC normalization method).
Embodiment 3
[0046] Take n-tetradecane (2.0 L) and water (4.8 mL), put them in a double-neck flask, 50 ° C, under stirring, drop PCl 3 (100.0 mmol), stirred for 24 h. At 100°C, add 2-(1 H -1-imidazolyl)-acetic acid (20.0 mmol). to be 2-(1 H -1-imidazolyl)-acetic acid is completely dissolved, at 80 ℃, drop into PCl 3 (160.0 mmol), stirred at 80°C for 24 h. Add 0.4mol / L hydrochloric acid (0.5 L), and react at 100°C for 24 h. After cooling to room temperature, the organic layer was decanted. After the aqueous layer was concentrated under reduced pressure, it was dropped into 250 mL of n-butanol at 50°C, and left to stand for crystallization at 20°C for 24 h, then filtered with suction. The filter cake was dried at 70°C for 5 h to obtain the target compound—zoledronic acid monohydrate (16.6 mmol, yield 83%, purity 99.7% by HPLC normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com