Method for extracting silver from silver-containing zinc concentrate and improving quality of zinc concentrate
A technology of zinc concentrate and zinc sulfide concentrate, which is applied to the improvement of process efficiency, photographic technology, instruments, etc., can solve the problems of silver dispersion loss, low recovery rate, high energy consumption, etc., and achieve low cost, high quality, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
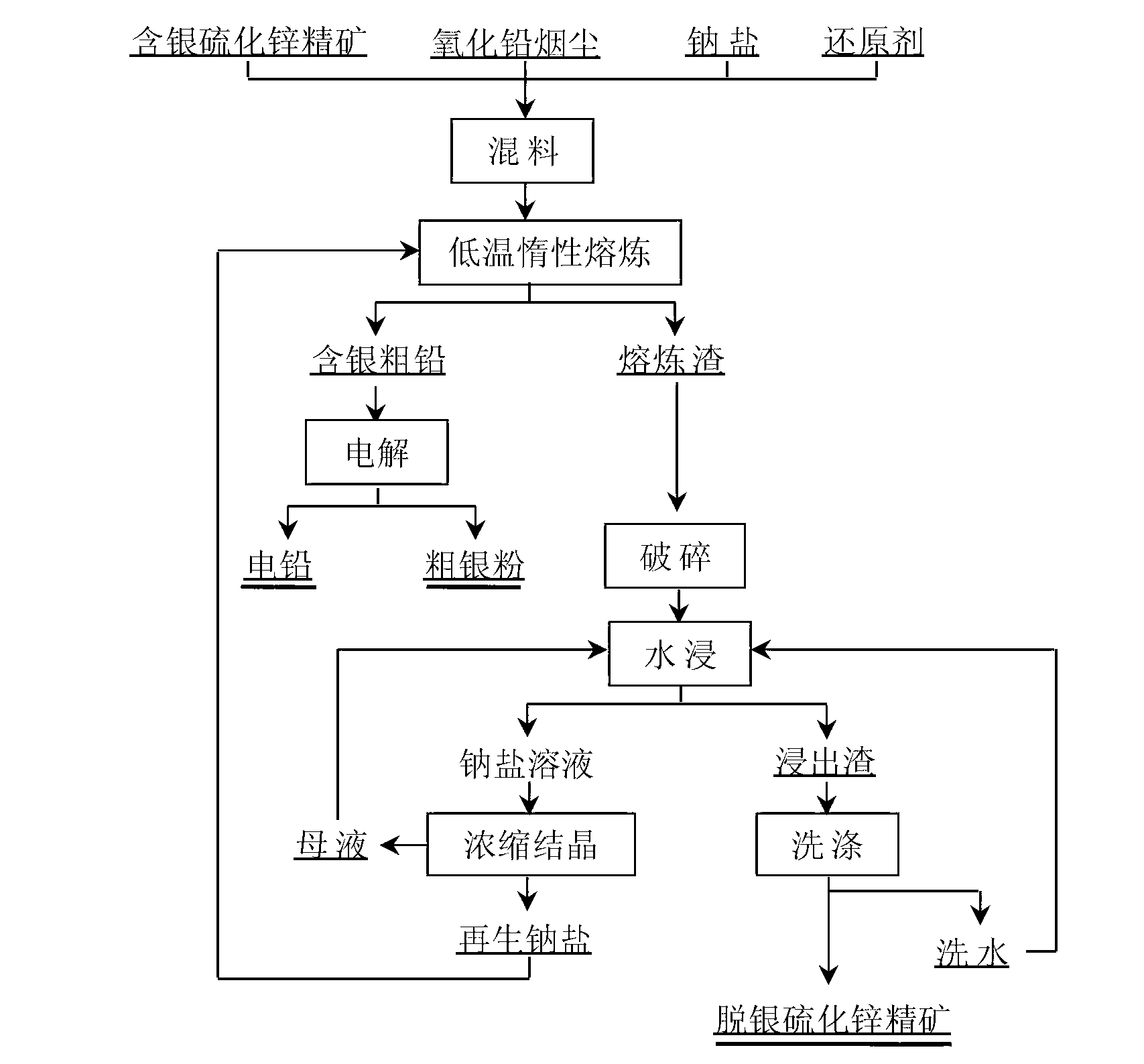
[0021] The chemical composition of a silver-containing zinc concentrate is (%): Zn44.5, Ag1.09, S29.14, Cu1.01, Pb0.83, Fe2.32, H 2 O4.6. First mix 100g of the silver-containing zinc concentrate with 100g of lead oxide fumes and 10.2g of pulverized coal, 250g of Na 2 CO 3 Fully mixed, the chemical composition of lead oxide dust is mainly (%): Pb74.36, Zn3.35, As0.72, Ag52g / t. The fully mixed material was reacted at 850°C for 120min. After the reaction, 75.21g of crude lead containing silver, 97.2% of crude lead grade, 1.01g of silver, and 93% of direct silver yield were obtained respectively after the reaction was finished. After the crude lead is separated, the smelting slag is leached with water, the leaching temperature is 75°C, the liquid-solid ratio of water and smelting slag is 5:1 (volume (ml) / mass (g)), and the leaching time is 30 minutes. After the leaching reaction, the liquid-solid separation was carried out, and the obtained leachate was evaporated and concentr...
Embodiment 2
[0023] The chemical composition of a silver-containing zinc concentrate is (%): Pb1.28, Zn39.72, As0.05, Fe8.20, S25.33, Ag726g / t. First the NaHCO that this silver-containing zinc concentrate of 67g and 33g lead oxide soot and 6g pulverized coal and embodiment 1 concentrated crystallization obtains 3150g is fully mixed, the chemical composition of lead oxide dust is mainly (%): Pb74.36, Zn3.35, As0.72, Ag52g / t. The fully mixed material was reacted at 870°C for 150min. After the reaction, 25.18g of silver-containing crude lead was obtained, the grade of crude lead was 98.1%, the silver content of smelting slag was 4g / t, and the direct silver recovery rate was 98%. After crude lead is separated, the smelting slag is leached with water, the leaching temperature is 60°C, the liquid-solid ratio of water to smelting slag is 4:1 (volume (ml) / mass (g)), and the leaching time is 60 minutes. After the leaching reaction, the liquid-solid separation was carried out, and the obtained lea...
Embodiment 3
[0025] The chemical composition of a silver-containing zinc concentrate is (%): Zn51.3, S27.46, Co0.0075, Pb2.83, Fe5.27, Cd0.119, As0.047, F0.004, Cl0.012, Ag1012g / t. First, mix 1000g of the silver-containing zinc concentrate with 1200g of lead oxide fumes and 180g of pulverized coal, 2000g of Na 2 CO 3 and 1000g NaOH are fully mixed, the chemical composition of lead oxide dust is mainly (%): Pb74.36, Zn3.35, As0.72, Ag52g / t. The fully mixed material was reacted at 890°C for 150min. After the reaction was finished, 927.6 g of crude lead containing silver, 98.1% of crude lead grade, 1.50 g of silver, and 92% silver yield were respectively obtained. After the crude lead is separated, the smelting slag is leached with water, the leaching temperature is 80°C, the liquid-solid ratio of water and smelting slag is 3:1 (volume (ml) / mass (g)), and the leaching time is 60 minutes. After the leaching reaction is finished, liquid-solid separation is carried out, and the obtained leac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com