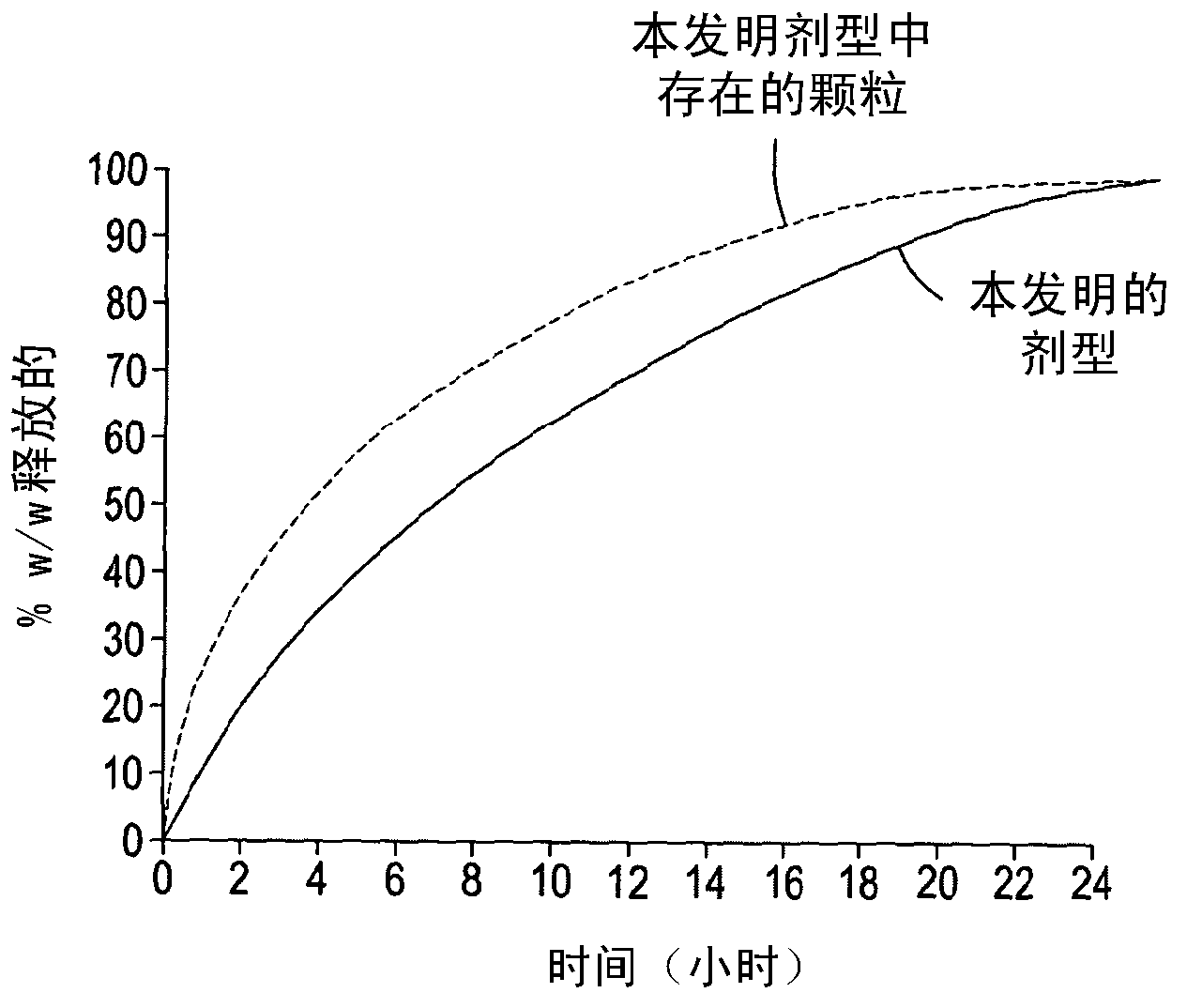
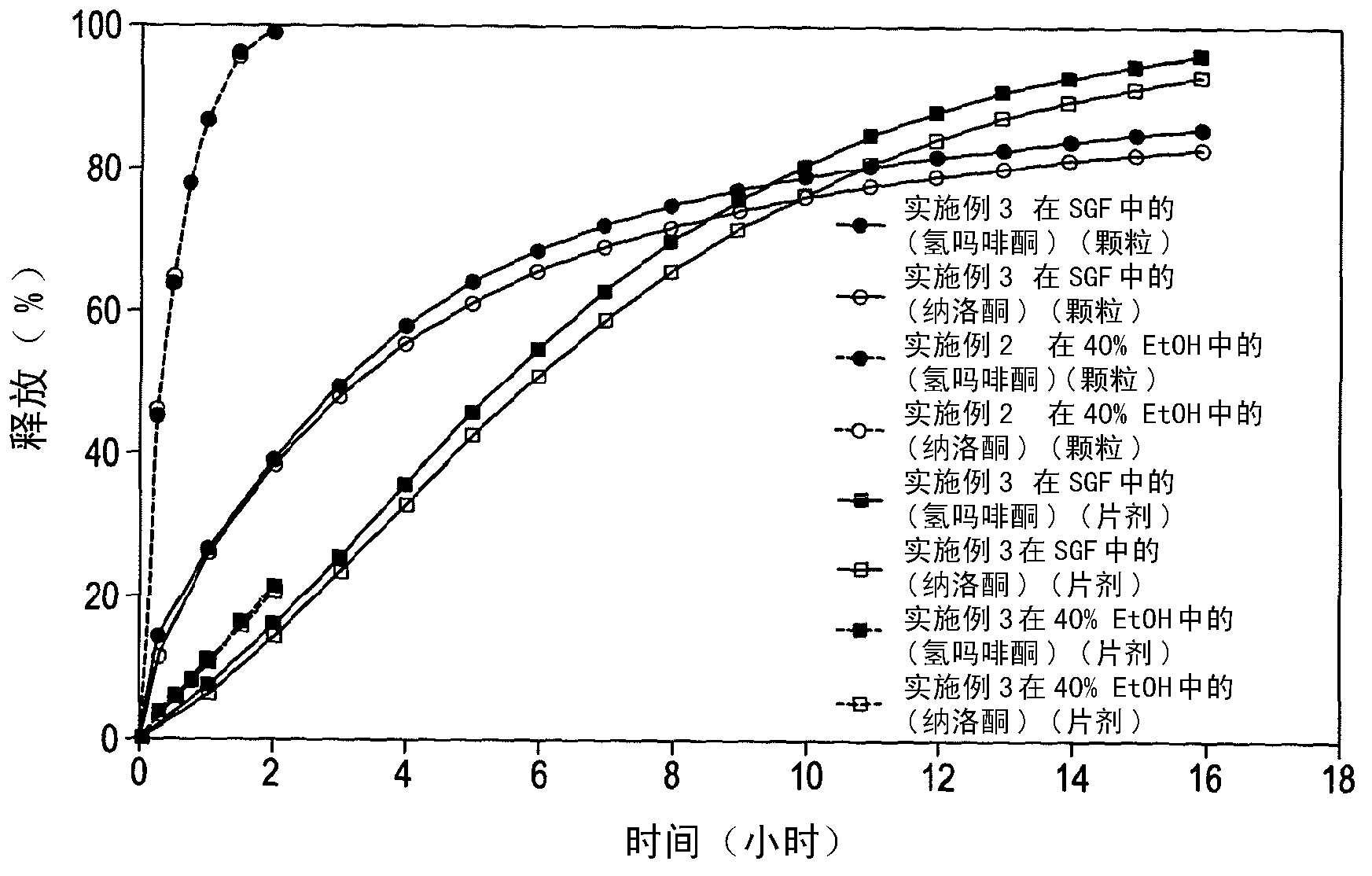
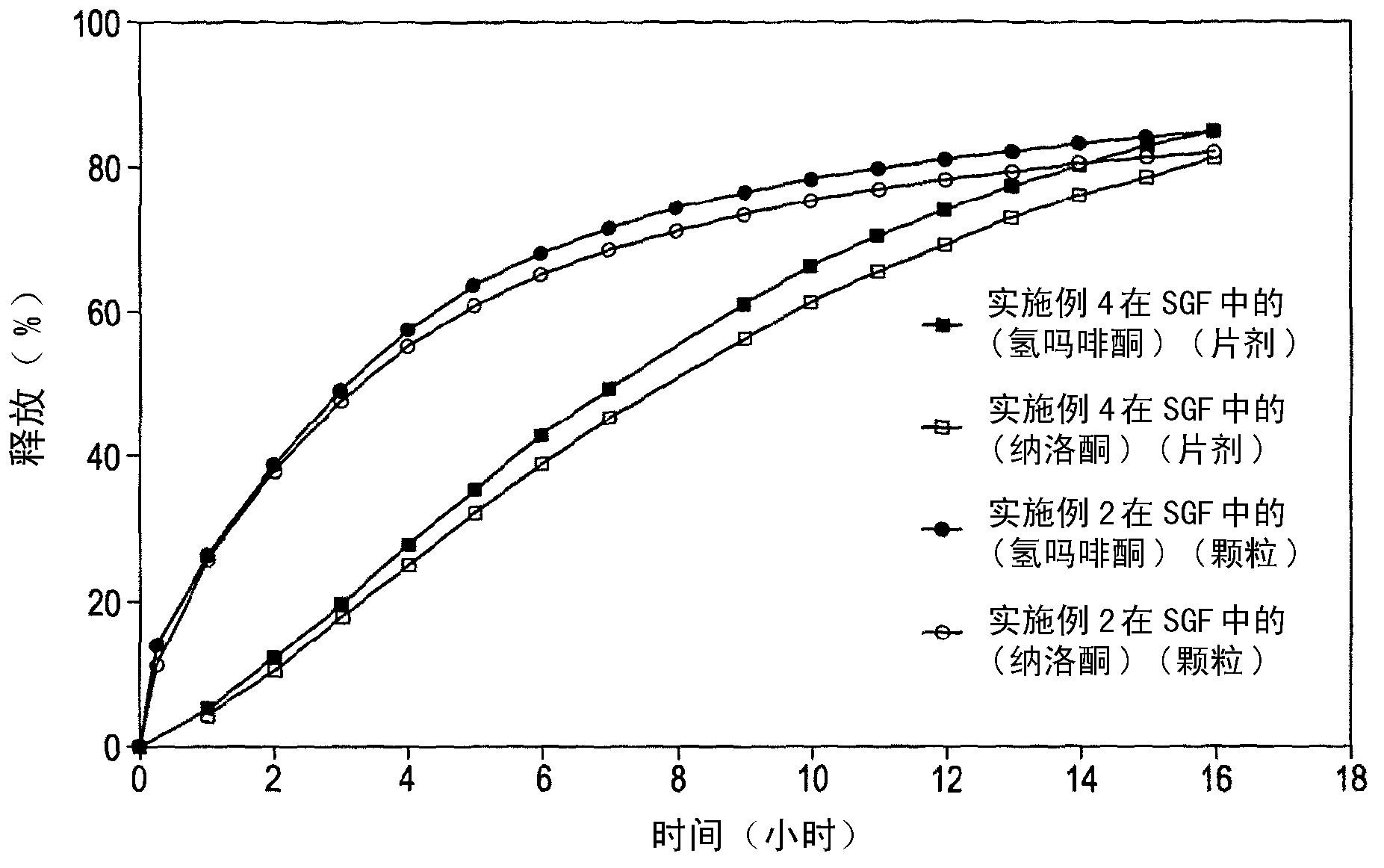
Dosage form
A dosage form and agonist technology, which is applied in the field of preparation of the dosage form and anti-interference dosage form, can solve the problems such as not easy to be crushed, large nasal administration, difficult to separate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] Drugs included in the preparation of dosage forms of the invention preferably have an average particle size of less than 500 microns, more preferably less than 300 microns, still more preferably less than 200 or 100 microns. There is no lower limit to the average particle size and may for example be below 50 microns, such as below 40 microns, below 30 microns, below 20 microns or even below 10 microns. The particle size of the drug can be determined by any conventional technique in the art, such as laser light scattering, sieve analysis, light microscopy or image analysis. In general, it is preferred that the largest dimension of the drug particle is smaller than the size of the granule (eg, smaller than the smallest dimension of the granule). It is also considered generally advantageous to utilize drug particles and non-melting and / or non-softening and / or non-dissolving excipients whose particle size is at least half the diameter of the molten extrudate and which In t...
Embodiment 1
[0217] Granules with the composition outlined in Table 1 were prepared as follows:
[0218]
[0219] *10% based on ethyl cellulose
[0220] **20% based on ethyl cellulose
[0221] The ethyl cellulose / triethyl citrate formulation is initially prepared by placing the ethyl cellulose in a mixer and adding the triethyl citrate gradually, for example by spraying. Mixing was continued until a homogeneous blend was obtained, then the mixture was allowed to stand overnight to allow the triethyl citrate to penetrate through the ethyl cellulose.
[0222] Hydromorphone hydrochloride, naloxone hydrochloride, stearyl alcohol, glyceryl dibehenate, and the ethylcellulose / triethyl citrate formulation prepared above were then added to the mixer and mixed. The resulting mixture is used The aqueous dispersion of NE 40D was granulated and the granules were subsequently dried to constant weight.
[0223] The dried granules are subsequently extruded. The melt extruder is set to predetermin...
Embodiment 2
[0230] Melt-extruded granules having the composition outlined in Table 3 below were prepared by first preparing placebo granules (by fluid bed granulation) having the composition outlined in Table 4 below, and secondly milling the placebo granules (using a 0.5 mm sieve Retsch mill), and third, blend the ground placebo granules with hydromorphone hydrochloride, naloxone hydrochloride, and magnesium stearate in an appropriately sized conical mixer to produce blended granules , and finally, the blended pellets were melt-extruded in a Leistritz Micro27 melt extruder to obtain extrudates, which were stretched and finally cut with a pelletizer to obtain melt-extruded pellets.
[0231] The particles obtained had an average diameter of 0.80 mm and an average length of 0.84 mm.
[0232] table 3
[0233]
[0234] S = solid content
[0235] Table 4
[0236]
[0237] S = solid content
[0238] Tablets with the composition outlined in Table 5 below were prepared by blending the g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com