Combined vaccine of seasonal influenza and pandemic influenza for people and preparation method
A technology for seasonal influenza and combined vaccine, which is applied in the field of combined seasonal influenza and pandemic influenza vaccine for human use and its preparation field, can solve the problems such as difficulty in large-scale vaccination of avian influenza vaccine, and achieves low production cost, simple method and large scale. social and economic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of combined vaccine
[0028] 1 Basic requirements
[0029] The production and verification facilities, raw materials and auxiliary materials, water, utensils, laboratory animals, etc. should meet the relevant requirements of the “general” in the 2010 edition of the Chinese Pharmacopoeia.
[0030] 2.1 Chicken embryos for production
[0031] The embryos for virus seed passage and preparation are derived from SPF chickens; the embryos for vaccine production should be from healthy chickens raised in closed houses, and the embryos of 9-10 days old without deformities, clear blood vessels, and mobile are selected.
[0032] 2.2 Virus seeds
[0033] 2.2.1 Name and source
[0034] Seasonal influenza strains are seasonal influenza A and B strains recommended and provided by the WHO; avian influenza strains are NIBRG-14 (A / Vietnam / 1194) recommended and provided by the World Health Organization (WHO) / 2004 (H5N1)) virus strain.
[0035] 2.2.2 Establishment of seed batch
...
Embodiment 2
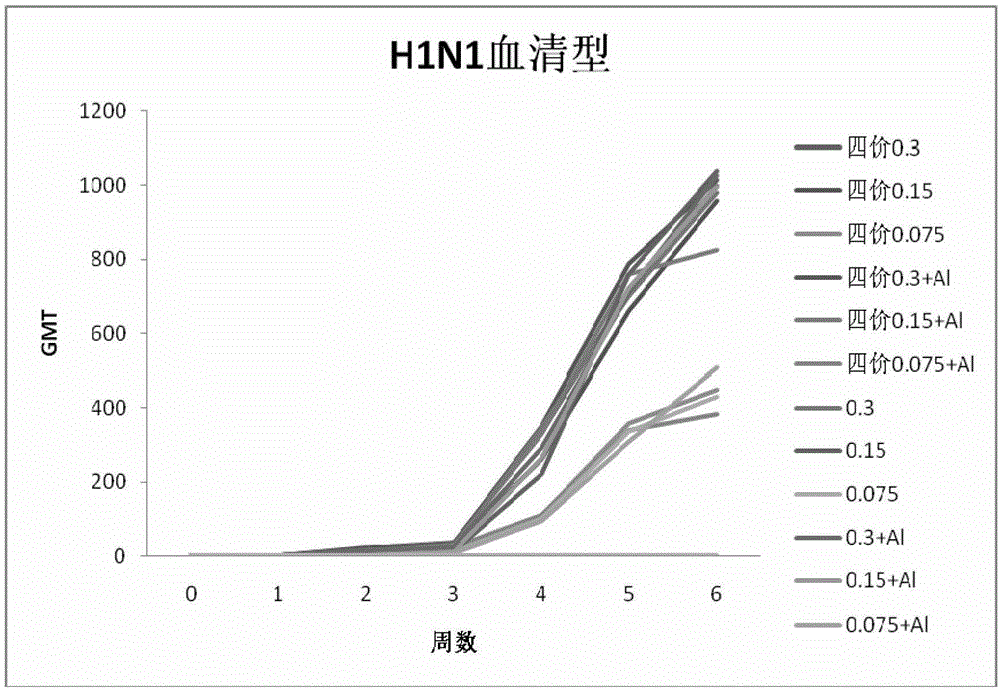
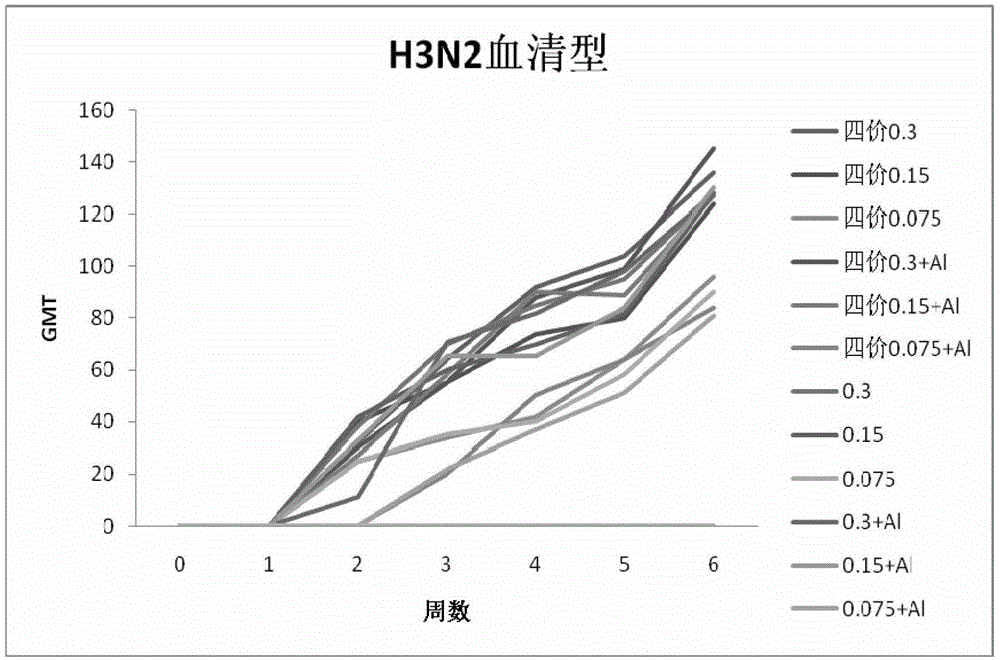
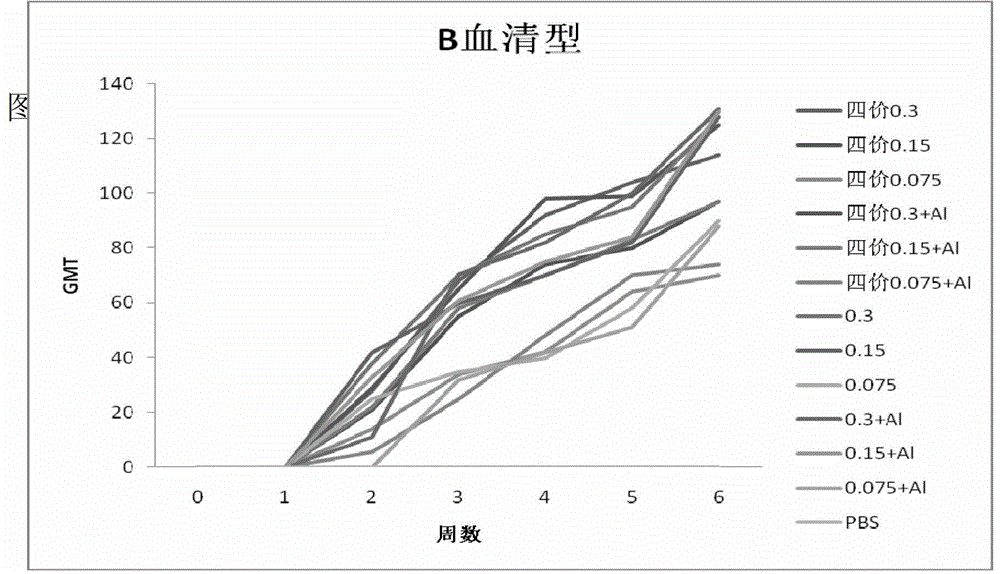
[0147] Example 2 Immunogenicity of vaccine
[0148] Using the combined vaccine and monovalent stock solution obtained in Example 1 as the antigen, SPF BABL / c mice were used as the animal model, and the immunogen of the vaccine was determined according to the changes in the titers of HA hemagglutination inhibitors of different serotypes in the sera of the mice after immunization Sex. The combined vaccine or monovalent vaccine stock solution was diluted to immunize mice. The experiment was divided into combined vaccine 0.3μg, 0.15μg, 0.075μg; combined vaccine plus aluminum adjuvant 0.3μg, 0.15μg, 0.075μg; H1N1, H3N2, B, H5N1 Type monovalent stock solution 0.3μg, 0.15μg, 0.075μg; H1N1, H3N2, B, H5N1 type monovalent stock solution plus aluminum adjuvant 0.3μg, 0.15μg, 0.075μg; PBS control group. Each group of 10 BALB / c mice aged 5 weeks, subcutaneously injected, 0.1ml / mouse, boosted once 21 days after the initial immunization, on the 0th day, 7th day, 14th day, 21st day, 28th day af...
Embodiment 3
[0168] Example 3 Safety of vaccine
[0169] According to the relevant provisions of the biological product registration classification and application data requirements in Annex 3 of the "Administrative Measures for Drug Registration", the combined vaccine has been passively implemented with reference to the provisions of the "Technical Guidelines for the Research on the Irritability, Allergy and Hemolysis of Chemical Drugs" Skin allergy test (PCA) and systemic active allergy test (ASA) to investigate animal allergy tests.
[0170] 1. Passive skin allergy test (PCA)
[0171] In this experiment, 300~400g guinea pigs were selected as experimental animals according to the nature of the antigen, and negative and positive control groups and different dose groups of the test substance were set up for subcutaneous immunization. Combination vaccine high-dose group: 15μg hemagglutinin antigen / mouse, combined vaccine low-dose group: 3μg hemagglutinin antigen / mouse, positive control: bovine s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com