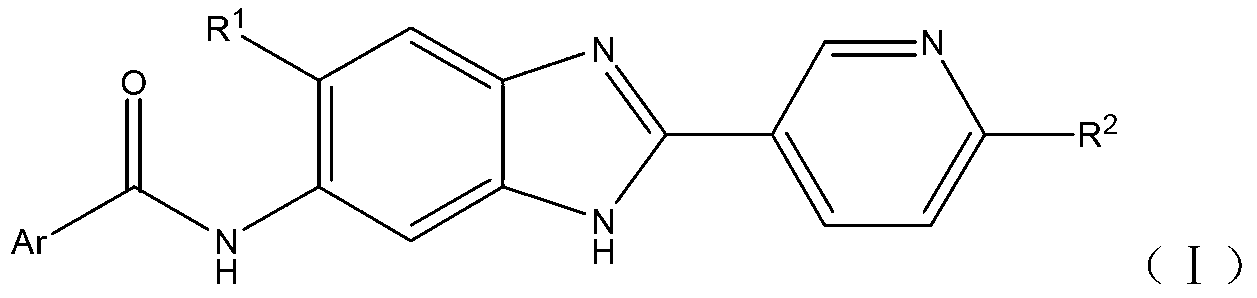
Benzimidazole amide compound as well as preparation method and application thereof
A technology of benzimidazole and compound, which is applied in the field of preparation of benzimidazole amide compounds, and can solve problems such as unreachable, high blood drug concentration, and ineffectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
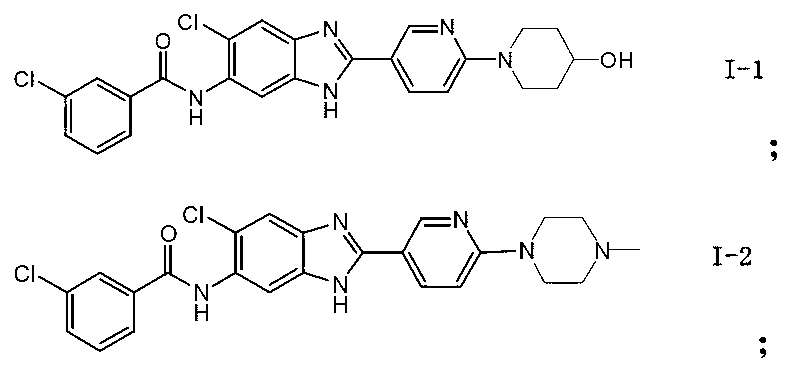
[0049] Synthesis of N-{5-chloro-2-[6-(4-hydroxypiperidinyl)(3-pyridyl]benzimidazol-6-yl}-3-chlorobenzamide (I-1)
[0050]
[0051] Experimental procedure
[0052] Compound 1 (20.0g) was dissolved in 600ml mixed solution (EtOH: H 2 O=5:1), ammonium chloride (20g) and acetic acid (20ml) were added, the system was heated to 60°C, and iron powder (32.4g) was added in batches. Keep at 60°C for 1 hour. The temperature was lowered, extracted with ethyl acetate, and spin-dried to obtain compound 2 (14 g, yield 85%).
[0053] Compound 2 (14.0g) was dissolved in pyridine (315ml), p-toluene chloride (39.3g) was added in batches, and the temperature was raised to 75°C for 1.5 hours. The reaction solution was spin-dried, dissolved in ethyl acetate, washed three times with 0.1N HCl aqueous solution, dried, and spin-dried to obtain compound 3 (29 g, yield 65.6%).
[0054] Compound 3 (21g) was dissolved in acetic acid (170ml), the reaction was heated to 70°C, the mixed solution (7ml of s...
Embodiment 2
[0065] synthesis
[0066] N-{5-chloro-2-[6-(4-methylpiperazinyl)(3-pyridyl]benzimidazol-6-yl}-3-chlorobenzamide (I-2)
[0067]
[0068] Experimental procedure
[0069] Compound 7 (500mg), N-methylpiperazine (488mg), N,N-diisopropylethylamine (1.26g) dissolved in N-methylpyrrolidone (20ml), added to a sealed tube under nitrogen protection React overnight at 130°C. The reaction solution was poured into ice water, extracted with ethyl acetate, washed with water five times, dried and spin-dried to obtain compound 12 (450 mg, yield 75%).
[0070] Compound 12 (450mg) was dissolved in EtOH / H2O=5 / 1 (96ml), ammonium chloride (450mg) was added, the system was heated to 60°C, and iron powder (408mg) was added in batches. Keep at 60°C for 1 hour. The temperature was lowered, extracted with ethyl acetate, and spin-dried to obtain compound 13 (200 mg, yield 48%).
[0071] Compound 13 (100mg), N,N-diisopropylethylamine (113mg), dissolved in (15ml) tetrahydrofuran, the reaction system...
Embodiment 3
[0073] synthesis
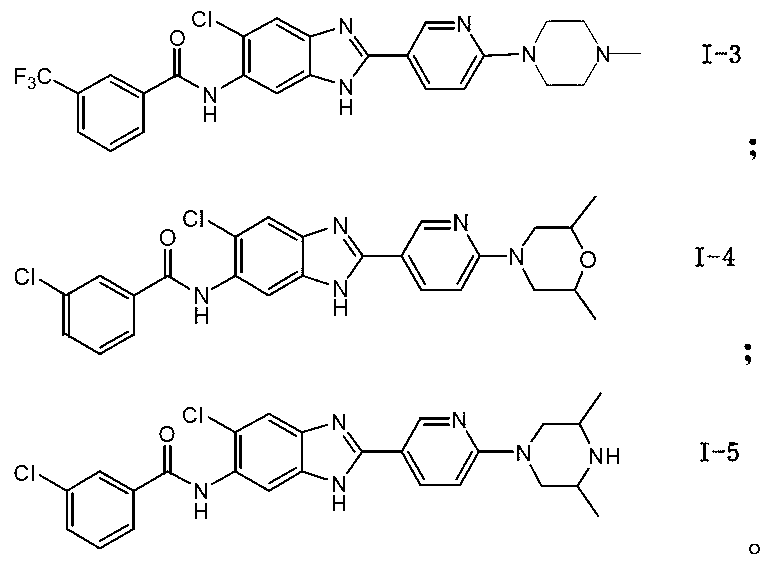
[0074] N-{5-chloro-2-[6-(4-methylpiperazinyl)(3-pyridyl]benzimidazol-6-yl}-3-trifluoromethylbenzamide (I-3)
[0075]
[0076] Experimental procedure
[0077] Compound 13 (100mg), N,N-diisopropylethylamine (113mg), was dissolved in (15ml) tetrahydrofuran, the reaction system was lowered to 0°C, and the mixed solution (72.6mg m-trifluoromethylbenzyl acid chloride / 5ml tetrahydrofuran), react at room temperature for 2 hours. Spin to dry, dissolve in ethyl acetate, wash with water, spin dry and separate by column chromatography to obtain compound I-3 (65 mg). LCM S=515.9(M+1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com