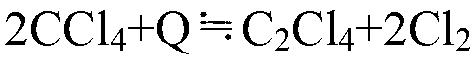Method for converting carbon tetrachloride to ethylene tetrachloride
A technology of tetrachloroethylene and carbon tetrachloride, applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation and other directions, can solve the problems of reducing the conversion amount of carbon tetrachloride, reducing the conversion amount of raw material carbon tetrachloride, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
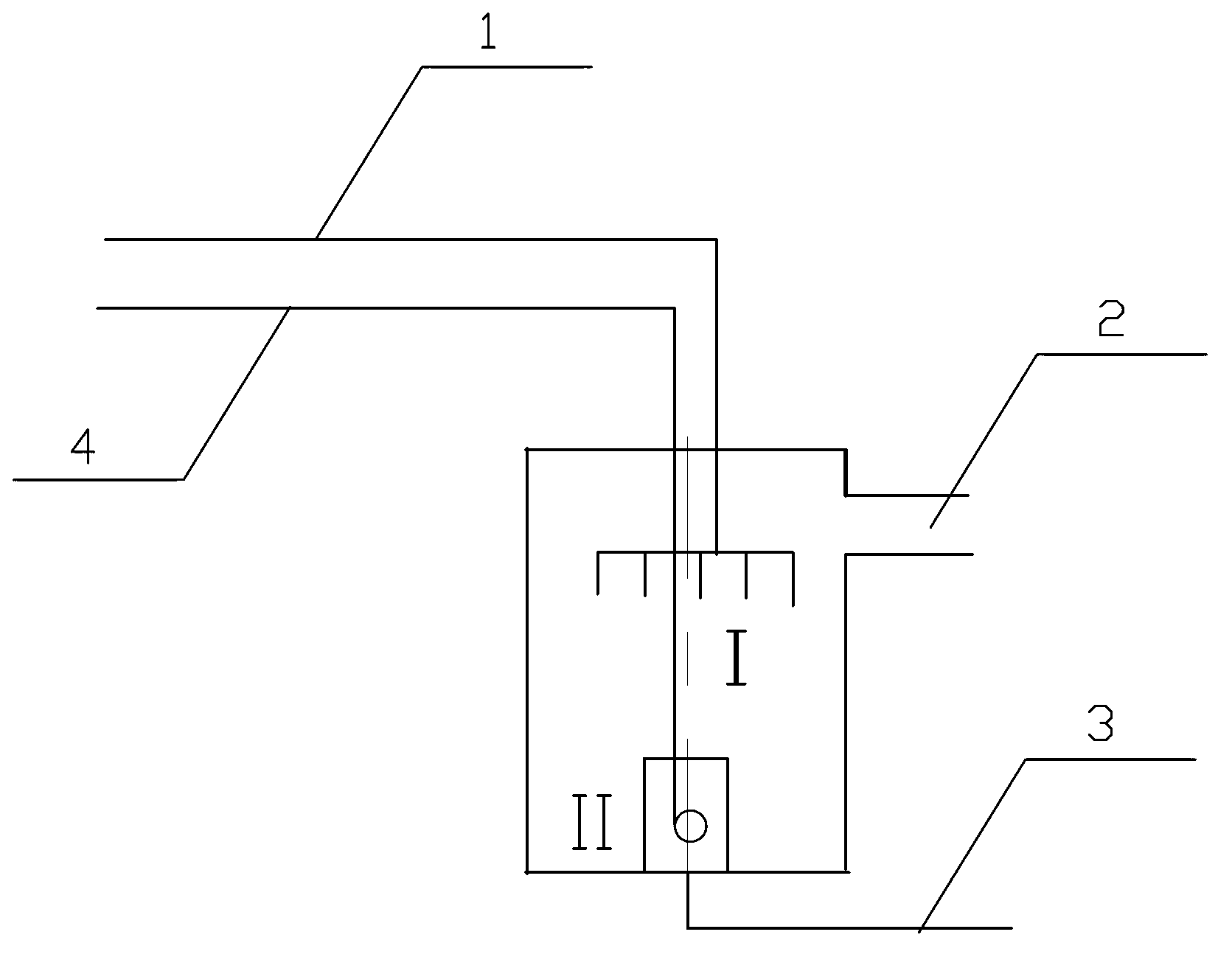
[0022] Such as figure 1 Shown, the method that described carbon tetrachloride is converted into tetrachlorethylene is as follows:
[0023] (1) First introduce hydrogen and oxygen combined gas 3 and excess vaporized chlorine gas 4 into combustion zone II at the bottom of the reaction furnace for combustion, keep the temperature of the reaction furnace at a certain temperature and pressure, and then introduce superheated carbon tetrachloride steam 1 to obtain four Chlorinated carbon, chlorine, tetrachloroethylene and hydrogen chloride perchlorethylene mixture2;
[0024] The superheated carbon tetrachloride steam is sprayed into the reaction zone I of the reaction furnace by injection to generate pyrolysis reaction. The spray speed of the distributor on the upper part of the reaction furnace is 49m / s, and the temperature of the superheated carbon tetrachloride steam is 74°C.
[0025] The carbon tetrachloride flow that introduces reaction zone I depends on the chlorine amount tha...
Embodiment 2
[0058] Step and reaction process are as implementing example 1, and superheated carbon tetrachloride steam adopts injection mode to be sprayed into reaction furnace reaction zone I and pyrolysis reaction takes place, and reaction furnace upper part distributor injection speed is at 43m / s, and the temperature of superheated carbon tetrachloride steam is 85°C.
[0059] The test results are as follows:
[0060] Feed, kg / h
[0061] Hydrogen: 4.5kg
[0062] Oxygen: 0.5kg
[0063] Chlorine: 83.9kg
[0064] Carbon tetrachloride: 363.8kg
[0065] Hydrogen / chlorine / carbon tetrachloride / oxygen (molar ratio) 1:1.05:2.1:0.11
[0066] Reaction conditions:
[0067] Temperature, 607°C
[0068] Pressure, 0.46kg / cm 2 (Gauge)
[0069] reaction product:
[0070] Produced tetrachlorethylene, 186kg / h;
[0071] Carbon tetrachloride converted into tetrachlorethylene, 344.9kg / h;
[0072] Excess amount of chlorine, the mole percentage of chlorine in the exhaust gas of the reactor: 0.05
...
Embodiment 3
[0076] Step and reaction process are as implementing example 1, and superheated carbon tetrachloride steam adopts injection mode to spray into reaction furnace reaction zone I and pyrolysis reaction takes place, and reaction furnace upper part distributor jet velocity is at 54m / s, and the temperature of superheated carbon tetrachloride steam It is 96°C.
[0077] The test results are as follows:
[0078] Feed, kg / h
[0079] Hydrogen: 4.5kg
[0080] Oxygen: 0.9kg
[0081] Chlorine: 83.9kg
[0082] Carbon tetrachloride: 502.4kg
[0083] Hydrogen / chlorine / carbon tetrachloride / oxygen (molar ratio) 1:1.05:2.9:0.2
[0084] Reaction conditions:
[0085] Temperature, 618°C
[0086] Pressure, 0.76kg / cm 2 (Gauge)
[0087] reaction product:
[0088] Produced tetrachlorethylene, 256.4kg / h;
[0089] Carbon tetrachloride converted into tetrachlorethylene, 475.8kg / h;
[0090] Excess amount of chlorine, the mole percentage of chlorine in the exhaust gas of the reactor: 0.04
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com