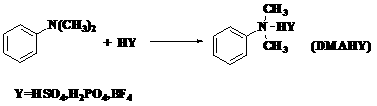
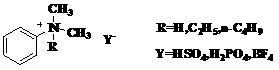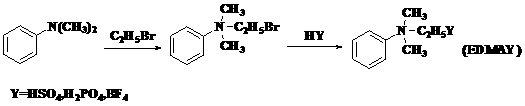Synthesis method of novel ionic liquid and application of novel ionic liquid to oil product denitrification
An ionic liquid and oil technology, applied in the petroleum industry, refined hydrocarbon oil, etc., can solve the problems of high cost of raw materials, large consumption, complicated preparation, etc. Simple and easy to use and operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: DMAHHSO 4 Synthesis
[0024] Synthesis steps: Add 0.2 mol of concentrated sulfuric acid dropwise to 0.22 mol of N,N-dimethylaniline under the condition of ice-water bath, the dropwise addition is completed within 30 min, under nitrogen protection, keep stirring at 50°C for 5 hours, and the product is mixed with ethyl acetate The ester and petroleum ether were washed three times successively, and the solvent was distilled off under reduced pressure.
[0025] DMAHHSO obtained by reaction 4 with V 乙醇 :V 乙酸乙酯 =5:7 mixed solution was recrystallized to obtain colorless crystals with a yield of 74.11%, and the measured melting point of the product was 89-91°C; 1 H-NMR (500MHZ, D 2 O): δ3.25(s,6H), 7.45(m,5H),7.97(s,1H)IR(KBr,ν / cm -1 ):ν3440 cm -1 ,3030cm -1 ,2921 cm -1 ,1598 cm -1 , 1496 cm -1 ,1466 cm -1 , 852 cm -1 .
Embodiment 2
[0026] Example 2: DMAHH 2 PO 4 Synthesis
[0027] The synthesis steps are the same as Example 1, only the reactant sulfuric acid is changed to phosphoric acid, and the reaction obtains DMAHH 2 PO 4 It is a colorless transparent liquid, which is a liquid with high viscosity at high temperature, and condenses into a colorless solid gel at room temperature, with a yield of 70.2%;
[0028] 1 H-NMR (500MHZ,D 2 O): δ3.24(s,6H), 7.43(m,5H),7.98(s,1H); IR(KBr,ν / cm -1 ):3453 cm -1 ,3032 cm -1 ,2970cm -1 ,1649 cm -1 ,1596 cm -1 ,1105cm -1 .
Embodiment 3
[0029] Example 3: DMAHBF 4 Synthesis
[0030] The synthesis steps are the same as in Example 1, only the reactant sulfuric acid is changed to fluoboric acid, and the obtained DMAHBF 4 It is a light green translucent liquid with good fluidity at high temperature. After cooling, a light green solid is obtained with a yield of 97%. Since the ionic liquid easily absorbs water in the air, the melting point of the solid cannot be accurately determined.
[0031] 1 H-NMR (500MHZ,D 2 O): δ3.25(s,6H), 7.44(m,5H),7.96(s,1H); IR(KBr,ν / cm -1 ):3006 cm -1 ,2921 cm -1 ,2856 cm -1 ,1599 cm -1 ,1497 cm -1 ,1084 cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com