Application of L-cysteine-enveloped nanogold in chiral recognition of tyrosine
A cysteine and chiral recognition technology, applied in the field of chiral recognition, can solve the problems of large reagent loss, low sensitivity, expensive chiral chromatographic column, etc., and achieve the effect of low cost and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The use of L-cysteine-coated gold nanoparticles in chiral recognition of tyrosine, the specific use method is as follows:
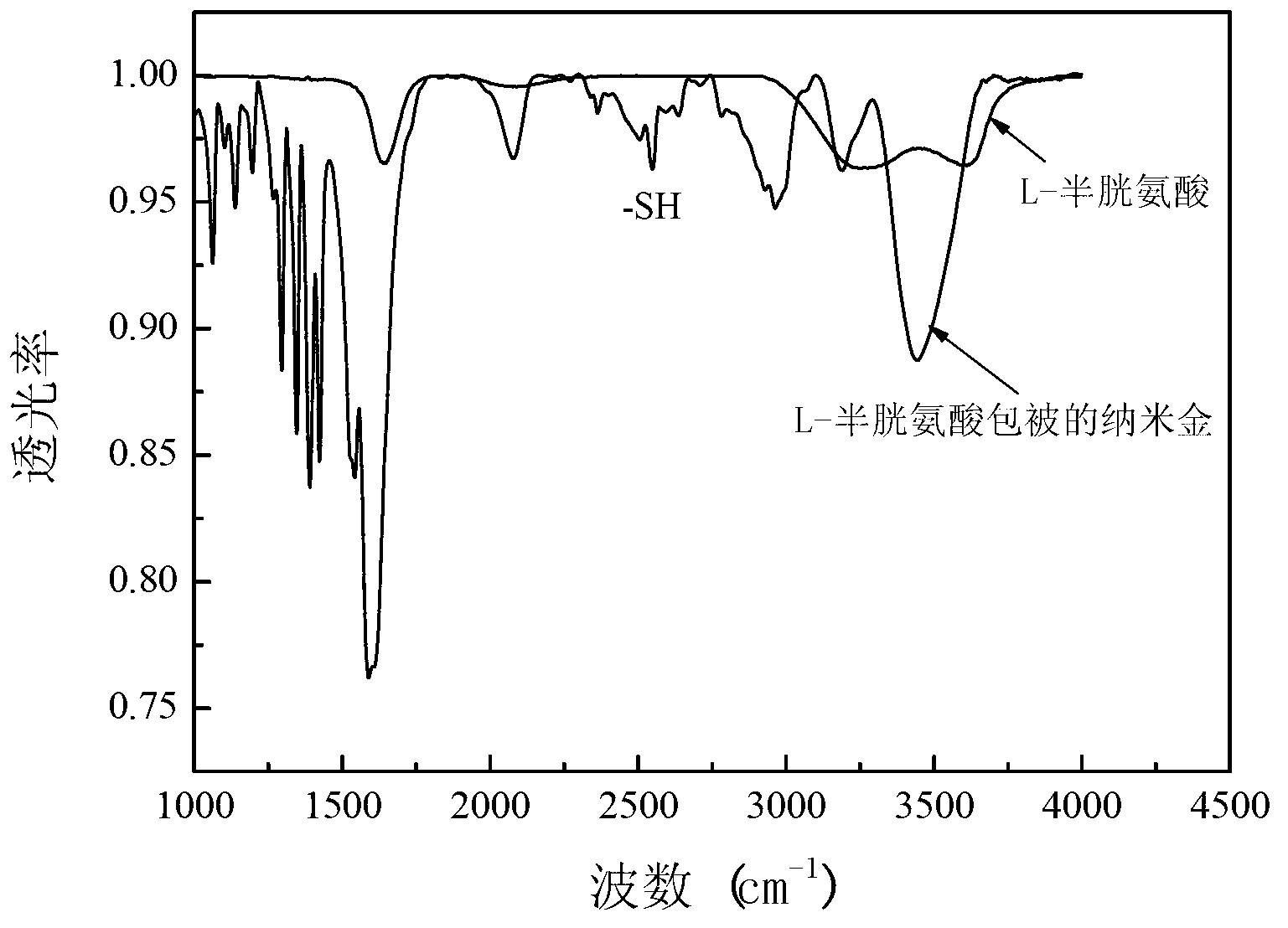
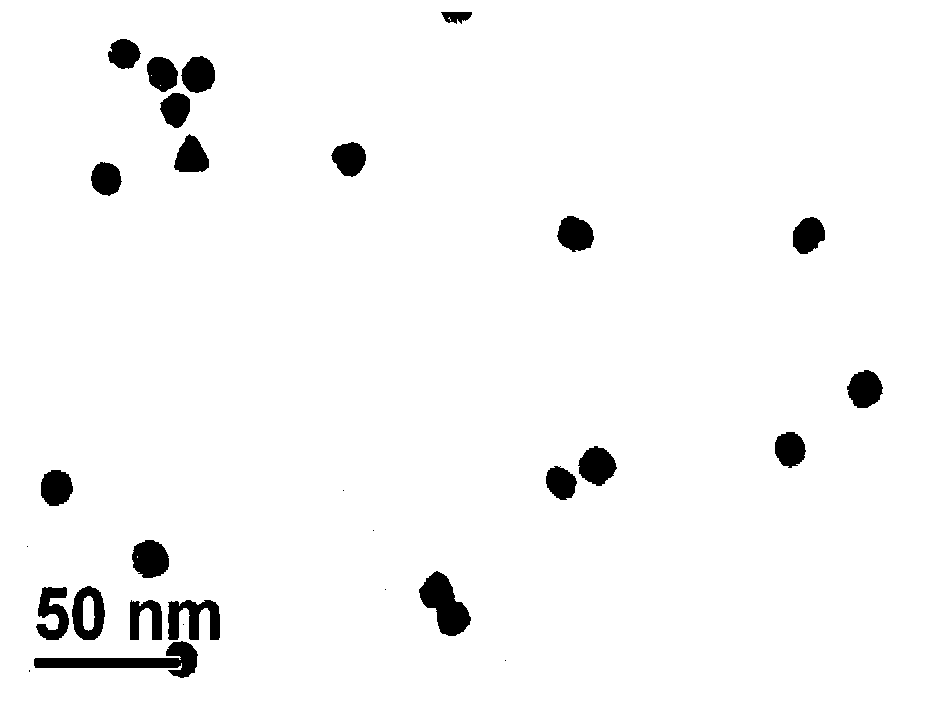
[0019] Heat and stir 50mL of chloroauric acid aqueous solution with a mass fraction of 0.04%, add 5.6mL of trisodium citrate aqueous solution with a mass fraction of 1% after boiling, the color of the solution changes from light yellow to purple, stir for 10 minutes, and the solution turns red , cooled to room temperature, and continued to stir for 13 minutes to prepare a nano-gold solution. Take 5 mL of nano-gold solution, add 100 μL of 2×10 -4 mol / L L-cysteine aqueous solution was stirred at room temperature for 2 hours to prepare L-cysteine-coated gold nanoparticles. The prepared L-cysteine-coated gold nanoparticles were characterized by Tensor27 infrared spectrometer and JEM-2100 transmission electron microscope. The results are shown in figure 1 and figure 2 . Depend on figure 1 It can be seen that after L-cysteine is coated with gol...
Embodiment 2
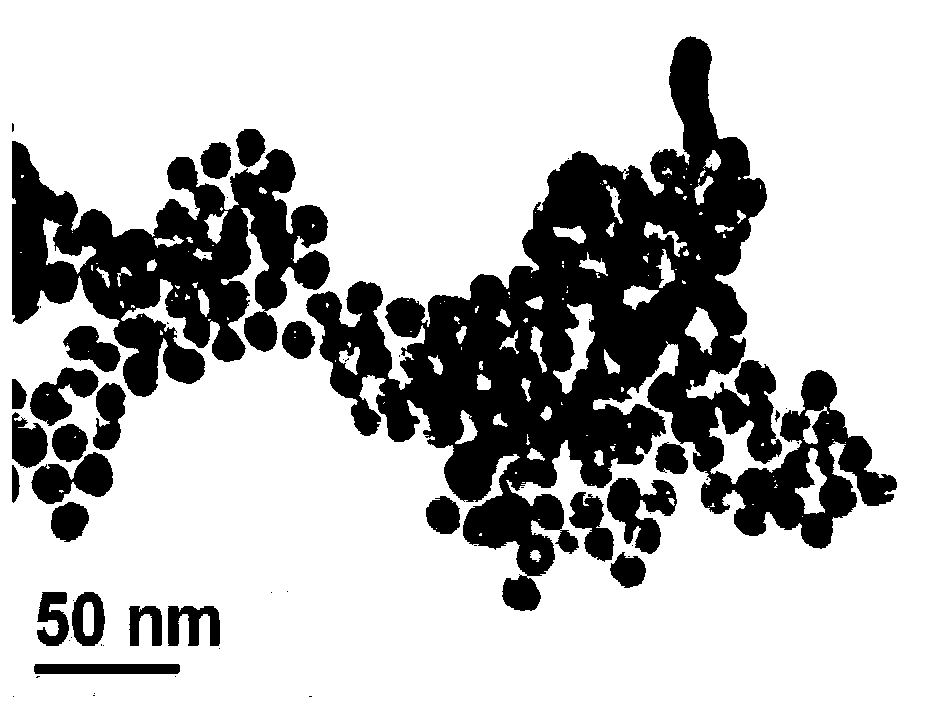
[0022] In Example 1, 120 μL of L-cysteine-coated gold nanoparticles, 300 μL of B–R buffer solution with a pH value of 4.0 were added to a 1.5 mL centrifuge tube, and then 80 μL of 2×10 -4 mol / L D-tyrosine aqueous solution, mixed evenly, the volume ratio of L-cysteine-coated nano-gold to B-R buffer solution, D-tyrosine aqueous solution was 1:2.5:0.67, static at room temperature Leave to react for 15 minutes, and the reaction solution is still red by naked eye observation. Depend on Figure 4 It can be seen that the intensity of the absorption peak of gold nanoparticles at 520nm has basically not changed, and no new absorption peaks have appeared, which is consistent with Figure 5 Most of the gold nanoparticles are still in a dispersed state, and only individual gold nanoparticles are connected to a small degree.
[0023] The preparation method of the L-cysteine-coated gold nanoparticles in this embodiment is the same as that in Example 1.
Embodiment 3
[0025] In Example 1, 120 μL of L-cysteine-coated gold nanoparticles, 240 μL of B–R buffer solution with a pH value of 4.0 were added to a 1.5 mL centrifuge tube, and then 60 μL of 1×10 -6 mol / L L-tyrosine aqueous solution, mixed evenly, the volume ratio of L-cysteine-coated gold nanoparticles to B-R buffer solution, and L-tyrosine aqueous solution was 1:2:0.5, and the volume ratio was 1:2:0.5. Leave it to react for 20 minutes, observe the color change of the reaction solution with the naked eye, and measure its absorbance A at the absorption wavelength of 520nm and 650nm by using a UV-visible spectrophotometer. After calculation, A 650 / A 520 =0.375, proved to be L-tyrosine.
[0026] The preparation method of the L-cysteine-coated gold nanoparticles in this embodiment is the same as that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com