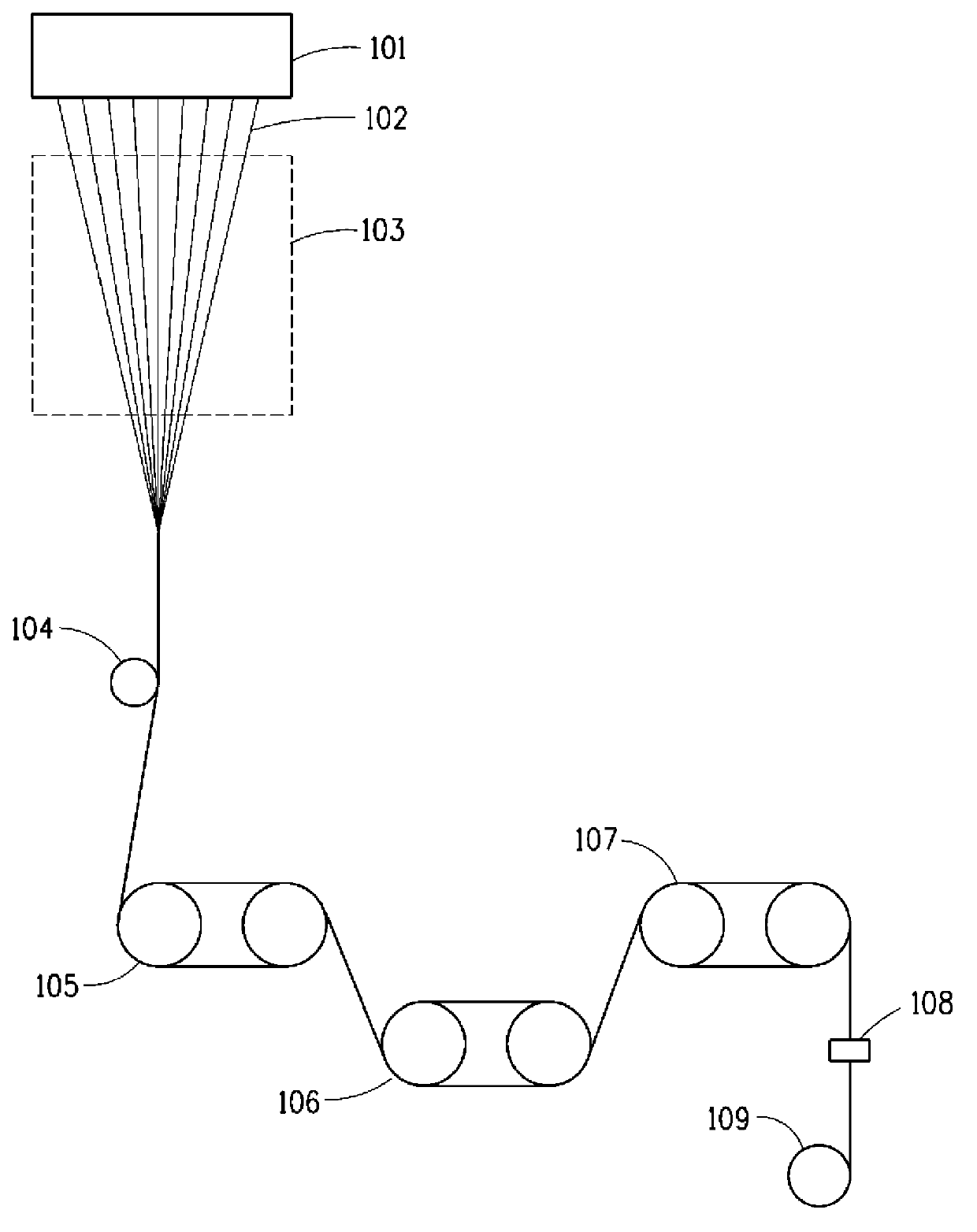
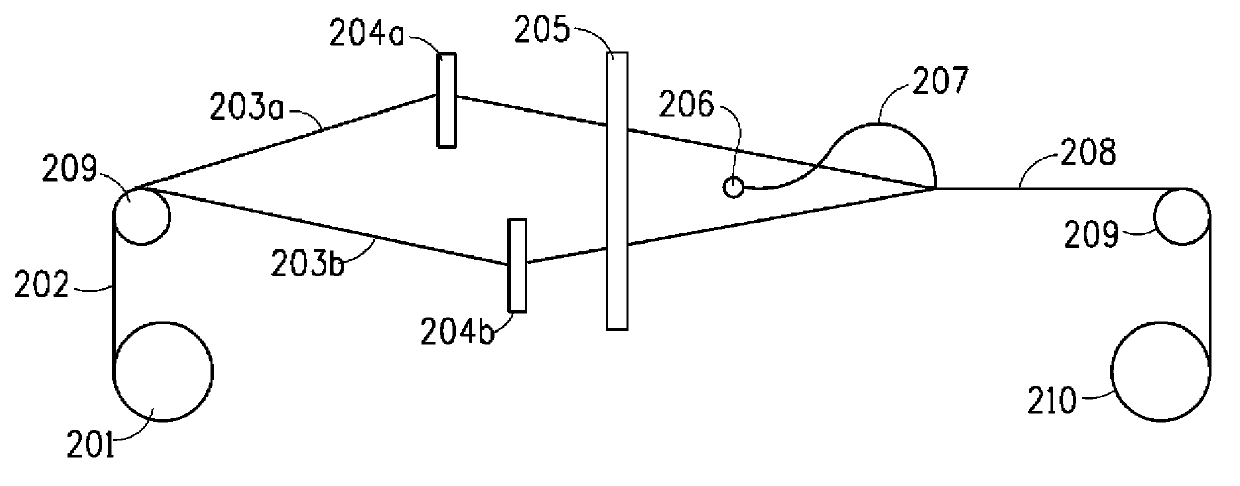
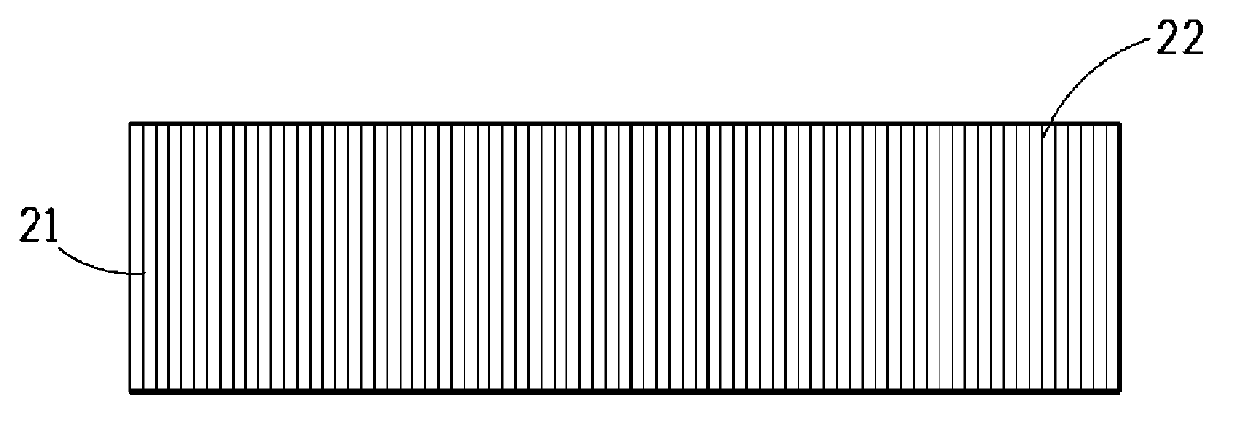
Carpets prepared from yarns comprising fluorinated polyester blend
A technology for carpets and yarns, applied in the direction of carpets, carpets, single-component polyester artificial filaments, etc., which can solve the problems of uneven processing quality and appearance decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1、2 and comparative example A
[0345] A. Dimethyl 5-(1,1,2-trifluoro-2-(perfluoropropoxy)ethoxy)isophthalate (F 10 -iso) synthesis :
[0346]
[0347] In a nitrogen purged dry box glovebox, THF (500 mL) and dimethyl 5-hydroxyisophthalate (42 g, 0.20 mol) were added to an oven dried round bottom reaction flask equipped with a stirrer and addition funnel middle. Potassium carbonate catalyst (6.955 g, 0.0504 mol) was added via addition funnel to form a reaction mixture. PPVE (79.8 g, 0.30 mol) was then added via addition funnel, and the resulting reaction mixture was heated to reflux at 66°C for 16 hours. The catalyst was then removed from the resulting mixture by filtration through a bed of silica gel. The resulting filtrate was concentrated in vacuo using a rotary evaporator followed by vacuum distillation to obtain 81.04 g (85.12% yield) of the desired 5-(1,1,2-trifluoro-2-( Perfluoropropoxy)ethoxy)dimethylisophthalate (F 10 -iso).
[0348] B.F. 10 -iso with 50 mol% concentration of ...
example 3、4 and comparative example B
[0365] A. (5-(1,1,2-trifluoro-2-(1,1,2,3,3,3-hexafluoro-2-(perfluoropropoxy)propoxy)ethoxy) Dimethyl isophthalate (F 16 -iso) synthesis:
[0366]
[0367] The procedure of Example 1 Part A was repeated except that 129.6 g of PPPVE was used instead of PPVE in Example 1 Part A. 123.39 g (96.10% yield) of the desired product (5-(1,1,2-trifluoro-2-(1,1,2,3,3,3-hexafluoro-2-( Perfluoropropoxy) propoxy) ethoxy) dimethyl isophthalate (F 16 -iso).
[0368] B.F. 16 The copolymer (F 10 -iso-50-co-tere) preparation
[0369]
[0370] Dimethyl terephthalate (36.24g, 0.187mmol), F 16 -iso (120 g, 0.187 mol) and 1,3-propanediol (51.2 g, 0.672 mol) were charged to a pre-dried 500 mL three-neck round bottom flask equipped with an overhead stirrer and distillation condenser. A nitrogen purge was applied to the flask at 23°C and stirring was started at 50 rpm to form a slurry. While stirring, the flask was evacuated to 100 Torr, then filled with nitrogen N 2 Repressurize for a tota...
example 5 and 6 and comparative example C
[0384] Steps A-D of Example 3 were repeated to produce F prepared as described in Example 3 16 -A two-batch blend of iso with Sorona Bright, one batch having 1% by weight of F 16 -iso-50-co-tere (example 5), while a batch has 2% by weight of F 16 -iso-50-co-tere (Example 6).
[0385] Each blend was melt spun into yarn following the procedure of Example 3, Part E, except that the spinneret had 34 holes per circular cross-section, 0.010 inch diameter by 0.040 inch length. Use unblended Sample served as control (CE-C). The spinning conditions are shown in Table 6. The mechanical properties of the yarns are shown in Table 7.
[0386] The yarns so produced are especially suitable for the preparation of knitted, woven and nonwoven textile goods.
[0387]
[0388]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com