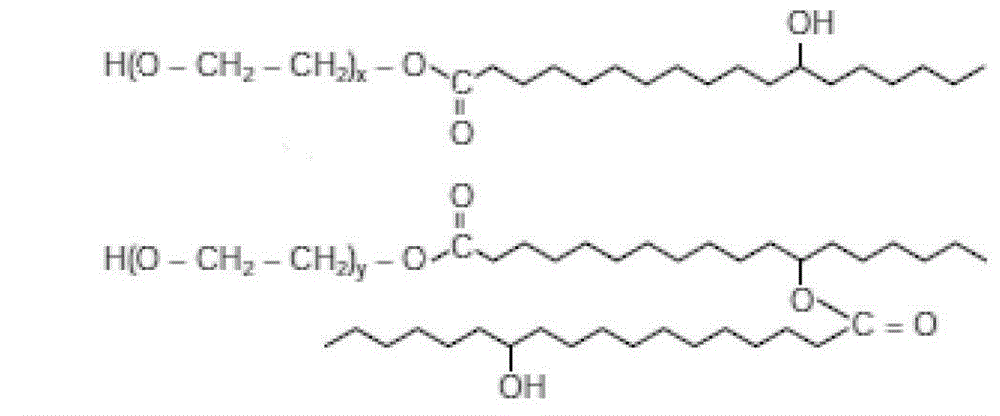
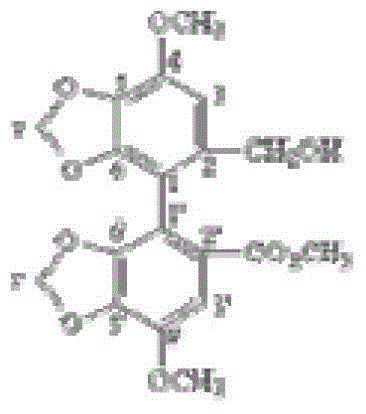
Bicyclol-containing water-soluble pharmaceutical composition and preparation, and preparation method thereof
A technology for water-soluble drugs and bicyclic alcohols, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, drug delivery, etc., can solve the problem of large amount of emulsifier, and achieve strong solubilization ability, convenient clinical medication, and low toxicity and side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The composition and dosage ratio are shown in Table 1.
[0048] Table 1
[0049] components
1000 consumption (g)
bicyclol
0.5
Solutol HS15
100
375
Add water for injection to
1500
[0050] The preparation method is as follows:
[0051] (1) The preparation process of the liquid medicine before freezing:
[0052] According to the dosage ratio shown in Table 1, add the solubilizer Solutol HS15 and bicyclic alcohol into the preparation container, and stir to dissolve under the heating condition of 50°C; add 80% water for injection, then add the proppant mannitol, and stir until completely dissolve, add water for injection to the full amount, and mix uniformly to obtain a water-soluble pharmaceutical composition containing bicyclic alcohol.
[0053] (2) Sterilize and filter the above-mentioned water-soluble pharmaceutical composition containing bicycloalcohol, and pack it into injection bottles, 1.5g / ...
Embodiment 2
[0060] The composition and dosage ratio are shown in Table 2.
[0061] Table 2
[0062] components
1000 consumption (g)
bicyclol
5
Cremophor RH40
40
PEG400
5
80
300
100
Add water for injection to
2000
[0063] The preparation method is as follows:
[0064] (1) The preparation process of the liquid medicine before freezing:
[0065] According to the dosage ratio shown in Table 2, add solubilizer Cremophor RH40, PEG400 and cosolvent ethanol, propylene glycol into the preparation container, stir and heat, and mix evenly; % of water for injection, then add proppant lactose, stir until fully dissolved, add water for injection to the full amount, and mix uniformly to obtain a water-soluble pharmaceutical composition containing bicyclic alcohol.
[0066] (2) Sterilize and filter the above-mentioned water-soluble pharmaceutical composition containing bic...
Embodiment 3
[0073] The composition and dosage ratio are shown in Table 3. For the preparation method, refer to the preparation method in Embodiment 1.
[0074] table 3
[0075] components
1000 consumption (g)
bicyclol
7.5
Solutol HS15
80
Poloxamer 188
30
150
600
100
Add water for injection to
1500
[0076] The preparation method is as follows:
[0077] (1) The preparation process of the liquid medicine before freezing:
[0078] According to the dosage ratio shown in Table 3, add solubilizer Solutol HS15, poloxamer 188 and cosolvent ethanol, glycerol into the preparation container, stir and heat, and mix well; Stir to dissolve; add 70% water for injection, then add proppant sorbitol, stir until fully dissolved, add water for injection to the full amount, and mix uniformly to obtain a water-soluble pharmaceutical composition containing bicyclol.
[0079] (2) Steril...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com