Low energy consumption propylene production technology
A production process and low energy consumption technology, applied in ethylene production, bulk chemical production, biological raw materials, etc., can solve the problems of high propylene yield and selectivity, achieve high selectivity, control temperature rise, and increase outlet temperature. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
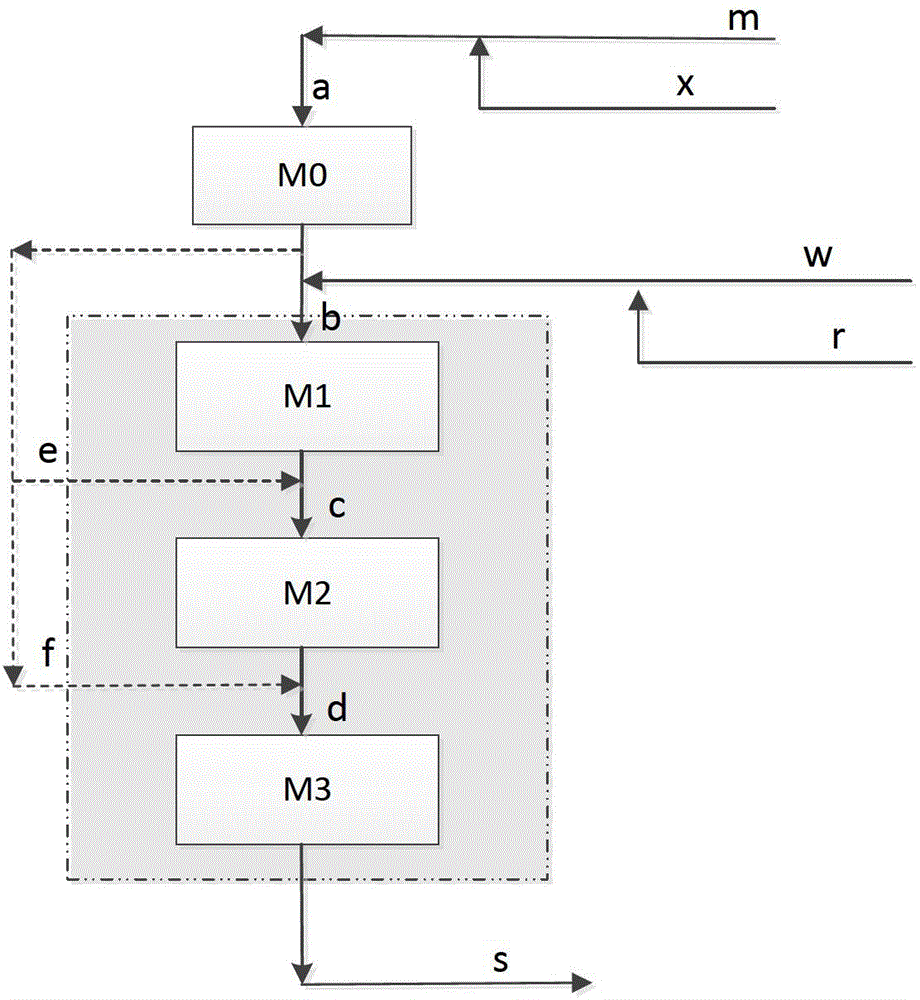
[0031] The catalyst used in this example is a ZSM-5 molecular sieve spherical or strip catalyst with a particle size of 1.0-3.0 mm. The raw material used is methanol, and the diluent gas is a mixture of methane and ethane. The molar ratio between the two is 2. :8, the molar ratio of diluent gas to methanol is 3:1.
[0032] The etherification reaction zone adopts a fixed bed reactor, the inlet temperature is 180°C, the outlet temperature is 220°C, the pressure is normal pressure, and the mass space velocity of methanol is 25h -1 . The molar ratio of refining hydrocarbons to primary products is 0.5:1, and the molar ratio of steam water to primary products is 4:1.
[0033] The OTP reaction zone adopts a fixed bed reactor with an inlet temperature of 440°C, operated under normal pressure, and a methanol mass space velocity of 12h -1 . The material in the reactor is contacted with the catalyst with 15% pre-coke, and the temperature of the outlet product stream is 480 ° C. After ...
Embodiment 2
[0036] The catalyst used in this example is ZSM-5 molecular sieve spherical catalyst with a particle size of 1.0-2.5 mm. The raw material used is methanol, the diluent gas is methane, and the molar ratio of diluent gas to methanol is 3:1.
[0037] The etherification reaction zone adopts a moving bed reactor with an inlet temperature of 230°C, an outlet temperature of 260°C, a pressure of 1.8MPa, and a methanol mass space velocity of 10h -1 . The output product of the etherification reactor is divided into two streams A and B, the mass ratio is 1:1, and the recycled hydrocarbon and steam water are mixed with the first stream of etherification reactor outlet product A, of which the recycled hydrocarbon and stream A The molar ratio of the mixture of methanol, dimethyl ether and water in stream A is 2.0:1, and the molar ratio of steam water to the mixture of methanol, dimethyl ether and water in stream A is 2:1.
[0038] The OTP reaction zone adopts two moving bed reactors, the i...
Embodiment 3
[0041] The catalyst used in this example is a ZSM-5 molecular sieve spherical catalyst with a particle size of 1.0-2.5 mm. The raw material used is methanol, and the diluent gas is methane and butane. The molar ratio of the three is 6:1 in sequence. The diluent gas and The molar ratio of methanol is 5:1.
[0042] The etherification reaction zone adopts a moving bed reactor with an inlet temperature of 260°C, an outlet temperature of 300°C, a pressure of 2.1MPa, and a methanol mass space velocity of 5h -1 . Divide the output product of the etherification reactor into three strands A, B, and C, with a mass ratio of 2:3:5, and mix the recycled hydrocarbon and steam water with the first etherification reactor outlet product A, in which the recycled hydrocarbon and The molar ratio of the mixture of methanol, dimethyl ether and water in stream A is 10:1, and the molar ratio of steam water to the mixture of methanol, dimethyl ether and water in stream A is 1:1.
[0043] The OTP rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com