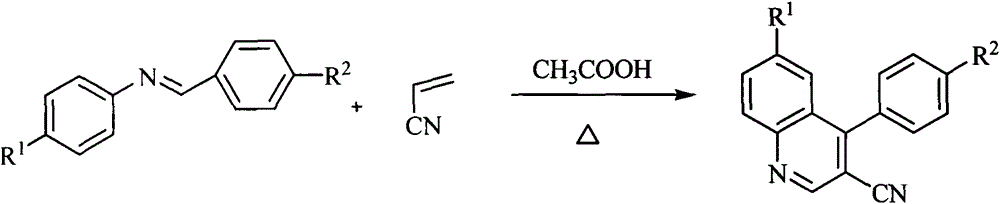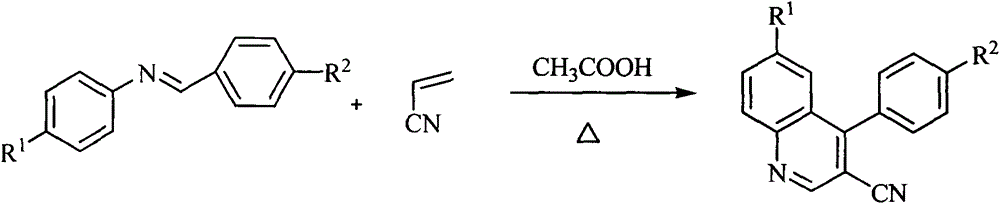A New Method for the Synthesis of Substituted Quinolines by the Reaction of Acrylonitrile and Substituted Phenyl Schiff Base
A technology of phenyl Schiff base and acrylonitrile, applied in the direction of organic chemistry, can solve the problems of heavy metal drug residues, unsuitable for wide application, difficulty in separation and recovery of transition metal catalysts, etc., and achieve the effect of simple operation and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] 1. Reaction steps (taking 3-cyano-4-phenylquinoline as an example):
[0015] 1. Add 0.545g of benzaldehyde-aniline Schiff base, 0.26g of acrylonitrile and 5.5g of glacial acetic acid into a 50ml round-bottomed flask equipped with a reflux condenser, stir evenly and heat the reaction to 105-110°C for a period of time Afterwards, the end point of the reaction was detected by TLC. After completion of the reaction, cool to room temperature, and decompress to remove CH 3 COOH, 0.49 g of 3-cyano-4-phenylquinoline was separated by thin layer chromatography.
[0016] In the thin layer chromatography adopted, a mixture of cyclohexane and ethyl acetate was used as an eluent, and the mixing volume ratio of cyclohexane and ethyl acetate was 10:1.
[0017] If the same process is used to replace the benzaldehyde-aniline Schiff base with different substituted Schiff bases, different substituted quinoline compounds can be obtained.
[0018] General reaction formula of the present in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com