Method for producing protein A by utilizing recombinant pichia pastoris
A technology of Pichia pastoris and recombinant yeast, which is applied in the field of bioengineering, can solve the problems of lack of large-scale fermentation and purification, low efficiency of exogenous protein secretion and expression, and limited application range, so as to achieve small quantity, reduce difficulty and cost, The effect of high affinity activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
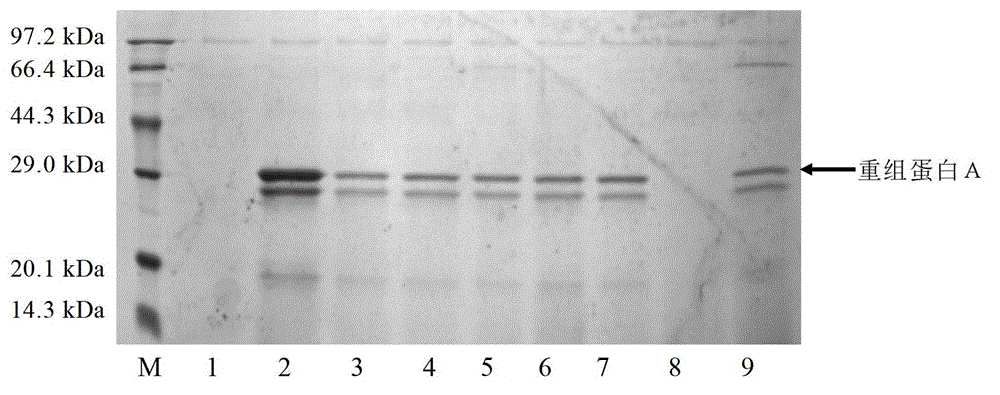
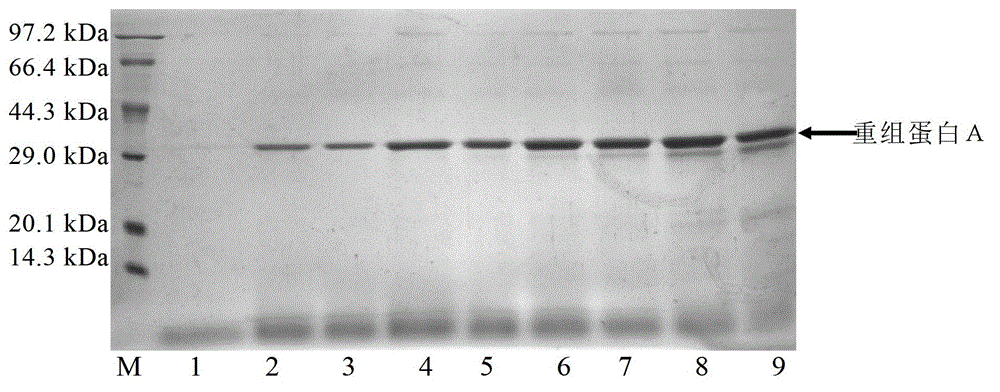
Image
Examples
Embodiment 1
[0039] 1. Construction and screening of recombinant Pichia pastoris strains expressing protein A
[0040] (1) PCR amplification of the target gene fragment
[0041] Using the recombinant plasmid (pET23a‐spa) with the protein A gene of Staphylococcus aureus as a template, primers were designed and restriction sites were added. Upstream primer (spa-a): 5'GCTGCA GAATTC GCG CAACACGATGAAG3' underlined sequence is EcoR I restriction site; downstream primer (spa‐b): 5'AGGTTTGTTG GCGGCCGC The underlined sequence TTATTTTGGT3' is the Not I restriction site. The reaction conditions were: pre-denaturation at 94°C for 5 min, followed by 35 cycles according to the following parameters: denaturation at 94°C for 30 s, annealing at 60°C for 40 s, and extension at 72°C for 2 min. The last cycle was extended at 72°C for 10 min.
[0042] (2) Ligation reaction
[0043] The PCR product was purified and recovered using a DNA purification / recovery kit (Bao Biological Dalian Co., Ltd.). The pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com