Novel method for hydrolyzing nitrile group to acylamino
A technology of amide group and new method, which is applied in the field of hydrolysis of nitrile group to amide group, which can solve the problems of easy side reactions, harsh reaction conditions, and unfriendly environment, and achieves cheap and easy-to-obtain catalysts, short reaction time, and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] 1. Reaction steps (take the conversion of benzonitrile into benzamide as an example):
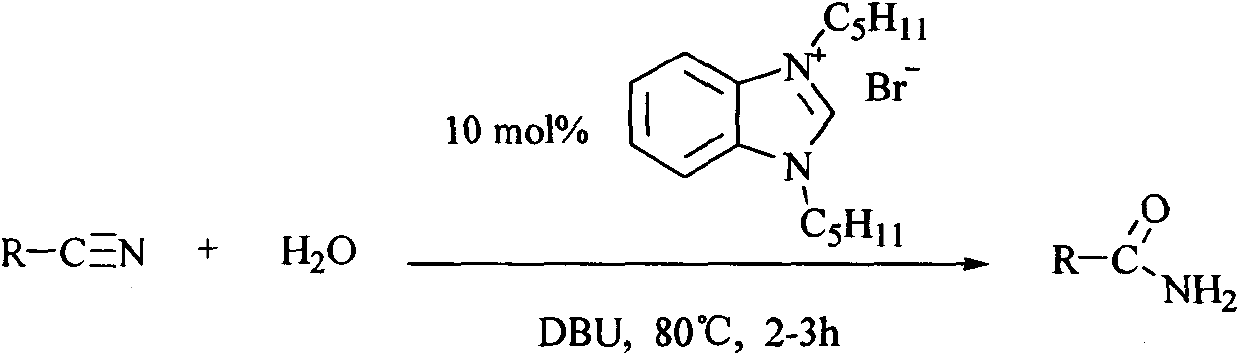
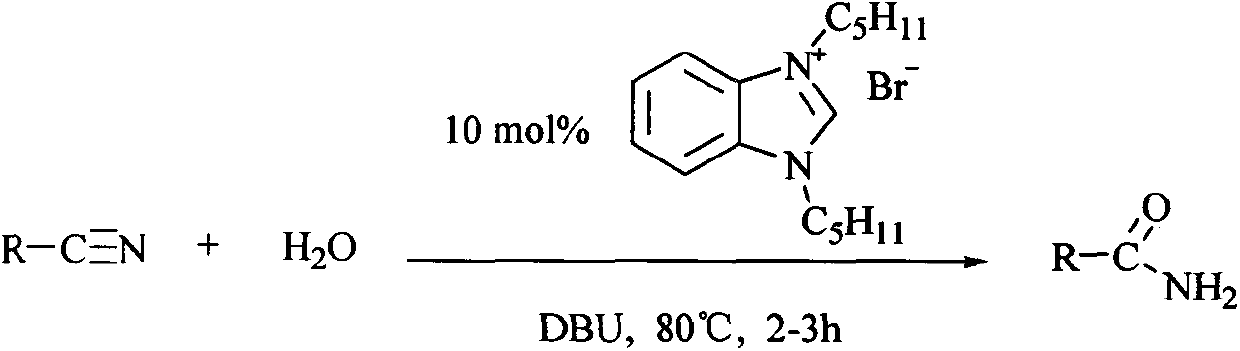
[0020] Add 2g of benzonitrile, 0.3g of N,N'-di-n-pentylbenzimidazolium bromide and 100ml of deionized water in a 25mL round-bottomed flask, and add 0.1g of DBU (1,8 -diazabicyclo[5.4.0]undec-7-ene), heated to 80°C, kept at reflux for 2.5h, after the reaction, most of the water was evaporated and thin-layer chromatography separated benzamide amine 1.9 g.
[0021] In the thin layer chromatography adopted, a mixture of cyclohexane and ethyl acetate was used as an eluent, and the mixing volume ratio of cyclohexane and ethyl acetate was 10:1.
[0022] Different types of amide compounds can be obtained by replacing benzonitrile with different substituted nitrile compounds.
[0023] General reaction formula of the present invention is:
[0024]
[0025] 2. Product identification:
[0026] The experimental data of the different substituted amides produced by the process of the present...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com