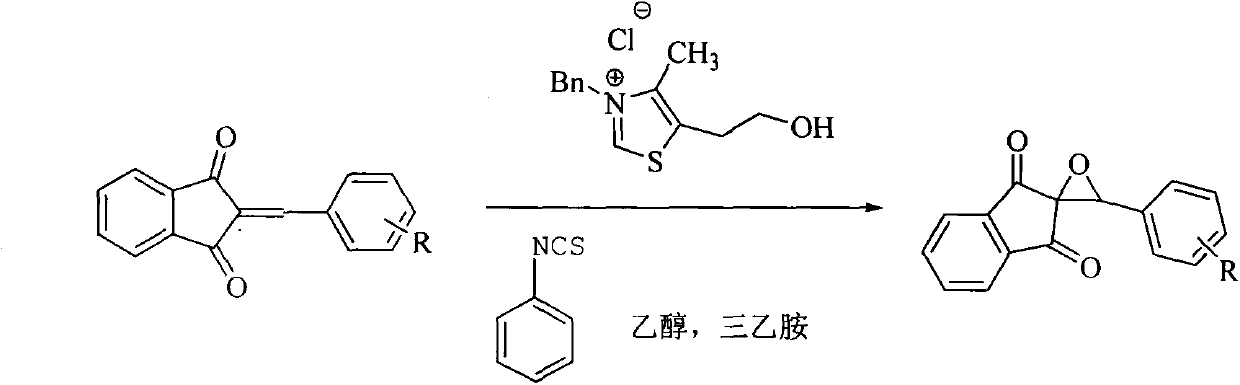
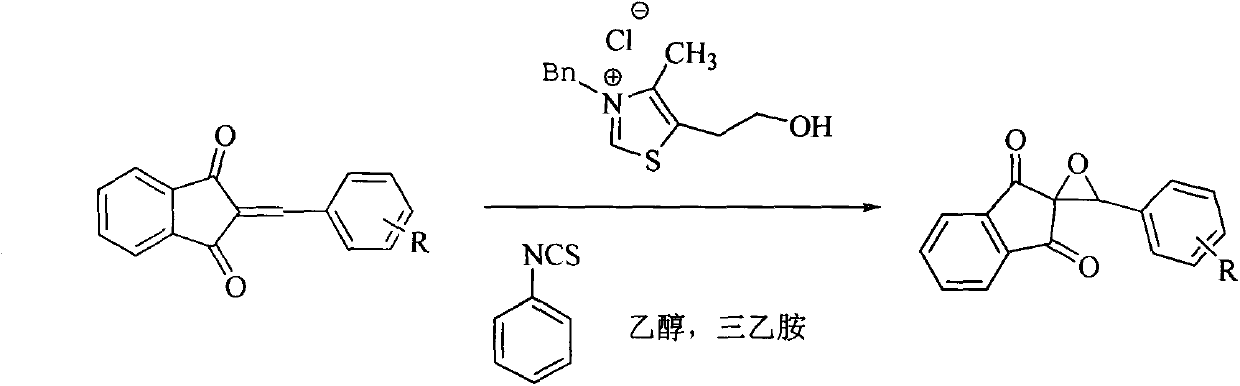
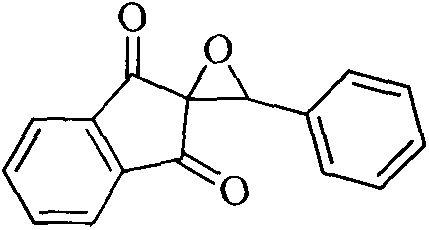
New epoxidation method of 2-benzylidene-1,3-indan diketone double bond
A technology of benzylidene and indandione, applied in the field of 2-benzylidene-1, can solve the problems of inability to synthesize epoxidation products, large damage to substrates, strong oxidation performance of hydrogen peroxide, etc., and achieves good effects , the effect of simple operation and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] 1. Reaction steps (taking the epoxidation of 2-phenylmethylene-1,3-indandione as an example):
[0014] Add 1mmol of 2-benzylidene-1,3-indandione, 0.25mmol of phenylisothiocyanate, 0.25mmol of thiazolium salt catalyst, 0.25mmol of triethylamine and 10mL of ethanol into a 50mL round bottom flask as solvent , stirred at room temperature for 30 min, evaporated most of the solvent residue and separated the product by thin layer chromatography (ethyl acetate:petroleum ether=1:4), product 227mg.
[0015] In the thin layer chromatography adopted, a mixture of cyclohexane and ethyl acetate was used as an eluent, and the mixing volume ratio of cyclohexane and ethyl acetate was 4:1.
[0016] Different epoxidized products can be obtained by substituting different substituted 2-benzylidene-1,3-indanediones for 2-benzylidene-1,3-indanediones.
[0017] General reaction formula of the present invention is:
[0018]
[0019] 2. Product identification:
[0020] Adopting different s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com