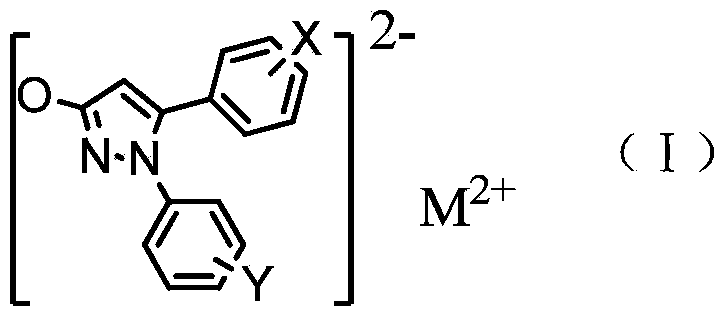Organic metal chelate, its preparation method and application
An organometallic and chelate technology, applied in the fields of botanical equipment and methods, applications, organic chemistry, etc., can solve the problems of poor bactericidal effect, high preparation cost and high toxicity of existing compounds, and achieve good protection and treatment. The effect of activity, simple preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
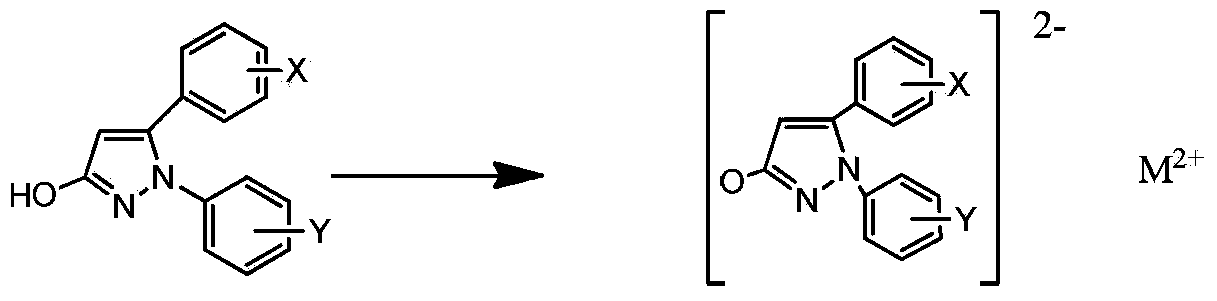
[0045] Under stirring, add 50ml of water to 1-phenyl-5-(4-methoxyphenyl)-3-hydroxypyrazole (0.05mol), add dropwise an aqueous solution of sodium hydroxide, stir at high speed, and wait until the solution is clear Then add a saturated copper chloride solution, react at 80°C for 3 hours, a solid precipitates out, cool to room temperature, stand and filter, wash with water, wash with ethanol, and dry to obtain 1-phenyl-5-(4-methoxy Phenyl)-3-hydroxypyrazole copper (compound 1) 4.59g.
[0046] 1 H-NMRδ: 3.79 (s, 6H), 6.80 (d, 2H), 7.03-7.39 (m, 18H).
[0047] ESI-MS: [M+H]=594.
Embodiment 2
[0049] Under stirring, add 50ml of water to 1-phenyl-5-(3,4,5-trimethoxyphenyl)-3-hydroxypyrazole (0.05mol), add dropwise an aqueous solution of sodium hydroxide, and stir at a high speed , after the solution is clarified, add a saturated zinc chloride solution, react at 100°C for 1 hour, a solid precipitates, cool to room temperature, stand and filter, wash with water, wash with ethanol, and dry to obtain 1-phenyl-5-(3 , 4,5-trimethoxyphenyl)-3-hydroxypyrazole zinc (compound 2) 5.58g.
[0050] 1 H NMRδ: 2.98 (s, 18H), 6.56 (d, 2H), 6.86 (d, 4H), 7.14-7.40 (m, 10H).
[0051] ESI-MS: [M+H]=715.
Embodiment 3
[0053] Under stirring, add 70ml of water to 1-(4-fluorophenyl)-5-(4-bromophenyl)-3-hydroxypyrazole (0.05mol), add dropwise an aqueous solution of sodium hydroxide, stir at high speed, After the solution is clarified, add saturated magnesium chloride solution, react at 80°C for 2 hours, a solid precipitates, cool to room temperature, stand and filter, wash with water, wash with ethanol, and dry to obtain 1-(4-fluorophenyl)-5- Magnesium (4-bromophenyl)-3-hydroxypyrazole (compound 3) 3.98g.
[0054] 1 H NMRδ: 6.06 (d, 2H), 6.96 (d, 4H), 7.11 (d, 4H), 7.14-7.40 (m, 8H).
[0055] ESI-MS: [M+H]=687.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com