Acidamide substitution podophyllum derivative with antineoplastic activity and preparation method and application thereof
A technology of anti-tumor activity and derivatives, applied in anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of high toxicity and side effects, poor bioavailability, limited use, etc. Tumor curative effect, antitumor activity improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
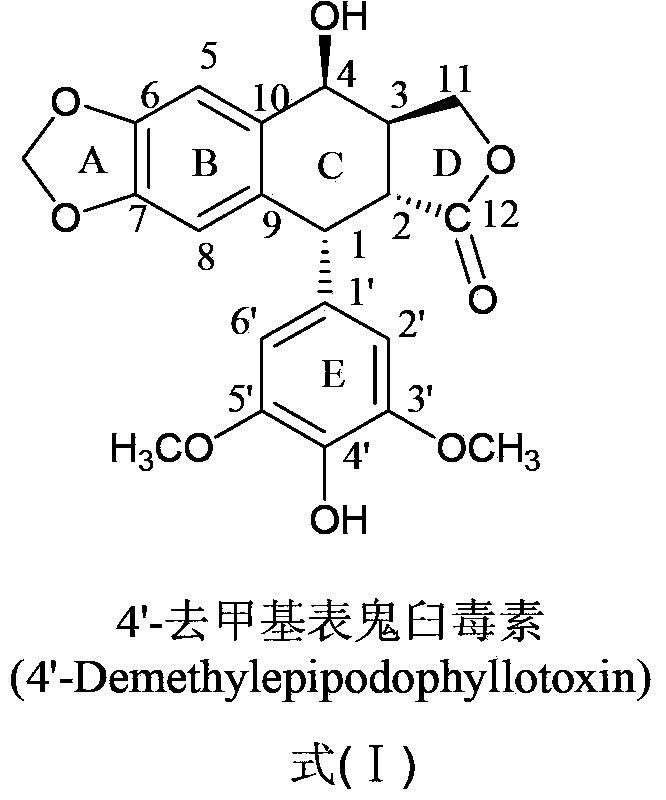
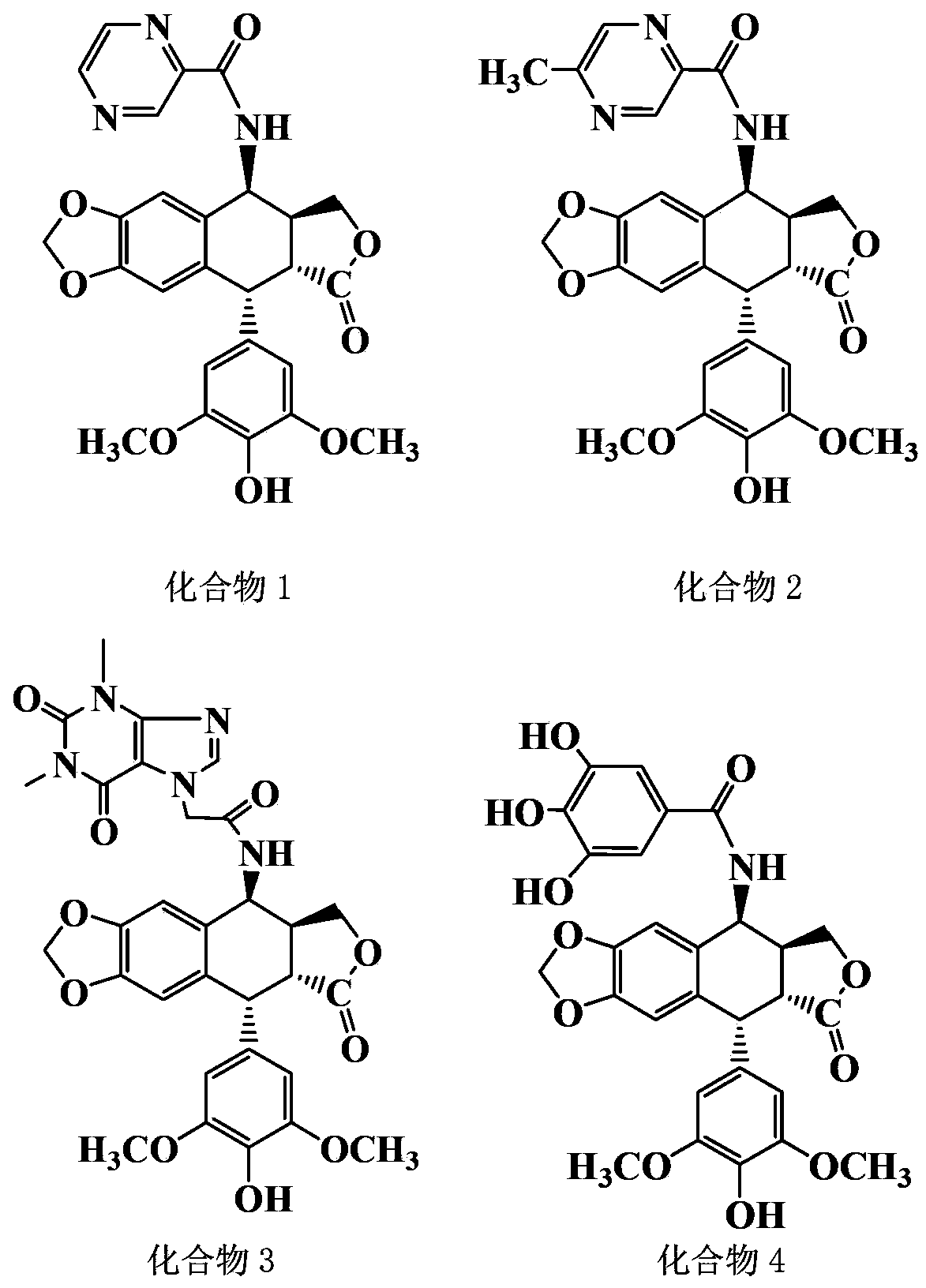
[0042] Example 14-Synthesis and purification of β-(2-pyrazinecarboxamide)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound 1)
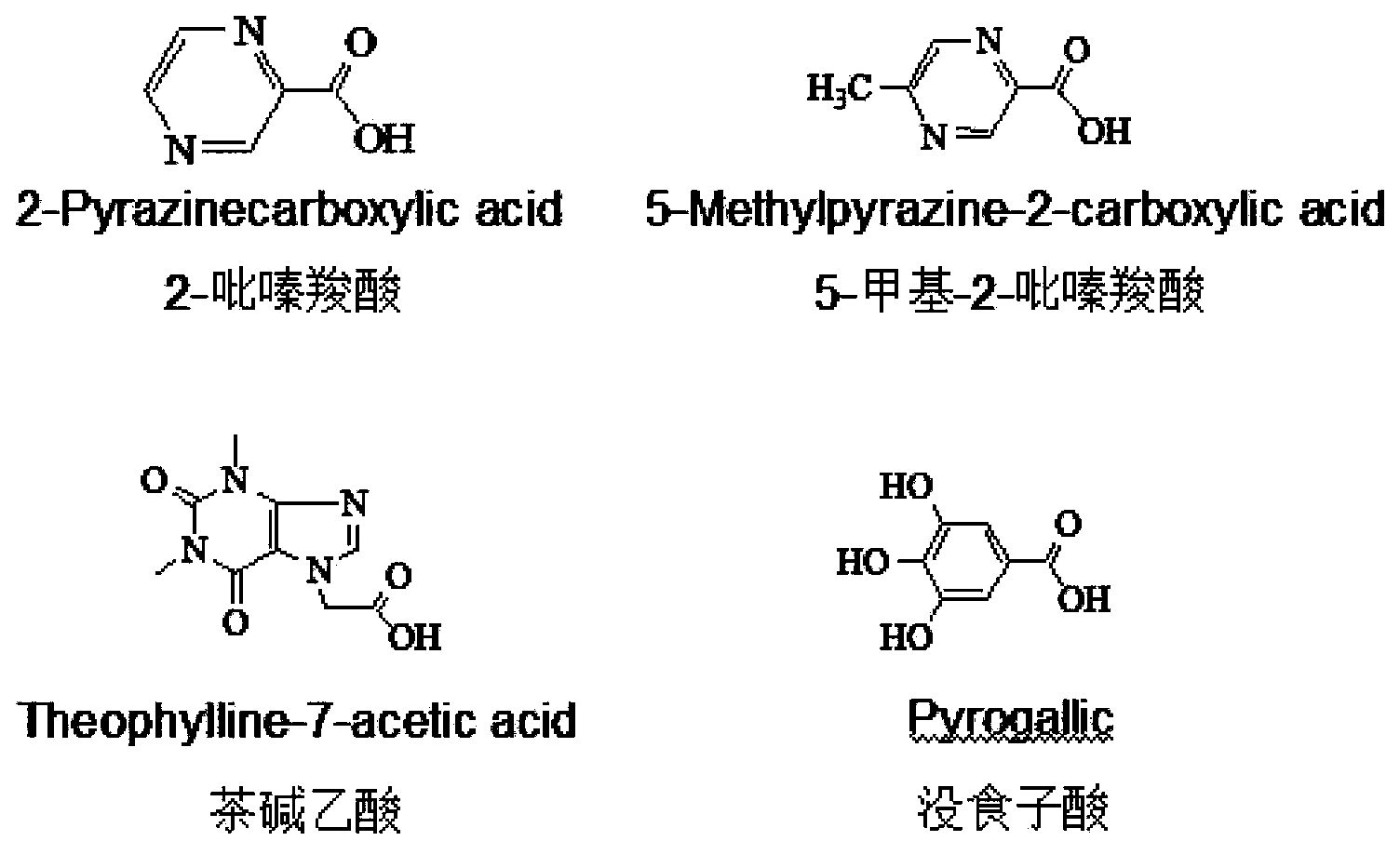
[0043] (1) Synthesis of 4-β-(2-pyrazinecarboxamide)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 124mg 2-pyrazinecarboxylic acid, 400mg4-β-NH 2 -4-Deoxy-4′-norepipodophyllotoxin, vacuum-dried at 45°C for 2 hours; under nitrogen protection, add the dried N,N-dimethylformamide into a four-necked bottle, and add 124 mg of the dried 2-pyrazinecarboxylic acid and 162mg of 1-hydroxy-benzo-triazole and 230mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, stirred and reacted in ice bath for 2~4h , and then under the protection of nitrogen, add dry 4-β-NH 2 - 4-deoxy-4'-norepipodophyllotoxin, slowly add triethylamine dropwise under stirring, remove the ice bath after the addition, and react at room temperature for 24-48 hours. After the reaction, extract the reaction solution with 20 mL of water, remove the organic phase of the lower layer...
Embodiment 24
[0049] Example 24-Synthesis and purification of β-(5-methyl-2-pyrazinecarboxamide)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound 2)
[0050] (1) Synthesis of 4-β-(5-methyl-2-pyrazinecarboxamide)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 138mg of 5-methyl 2-pyrazinecarboxylic acid, 400mg 4-β-NH 2 -4-Deoxy-4′-norepipodophyllotoxin, vacuum-dried at 45°C for 2 hours; under nitrogen protection, add the dried N,N-dimethylformamide into a four-necked bottle, and add 138 mg of the dried 5-Methyl-2-pyrazinecarboxylic acid and 162 mg 1-hydroxy-benzo-triazole and 230 mg 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride, under ice bath Stir the reaction for 2 to 4 hours, then add the dried 4-β-NH under the protection of nitrogen 2 - 4-deoxy-4'-norepipodophyllotoxin, slowly add triethylamine dropwise under stirring, remove the ice bath after the addition, and react at room temperature for 24-48 hours. After the reaction, extract the reaction solution with 20 mL of water...
Embodiment 34
[0056] Example 34-Synthesis and purification of β-(theophylline acetamide)-4-deoxy-4'-desmethyl epipodophyllotoxin (compound 3)
[0057] (1) Synthesis of 4-β-(theophylline acetamide)-4-deoxy-4'-desmethyl epipodophyllotoxin: Weigh 238mg theophylline acetic acid, 400mg4-β-NH 2 -4-Deoxy-4′-norepipodophyllotoxin, vacuum-dried at 45°C for 2 hours; under nitrogen protection, add the dried N,N-dimethylformamide into a four-necked bottle, and add 238 mg of the dried 5. Theophylline acetic acid, 162mg 1-hydroxy-benzo-triazole and 230mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride were stirred and reacted in an ice bath for 2-4 hours, and then Under nitrogen protection, add dry 4-β-NH 2 - 4-deoxy-4'-norepipodophyllotoxin, slowly add triethylamine dropwise under stirring, remove the ice bath after the addition, and react at room temperature for 24-48 hours. After the reaction, extract the reaction solution with 20 mL of water, remove the organic phase of the lower layer, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com