Isopimarane diterpenoid compounds and application thereof
A technology of isopimarane and compound, which is applied in the field of microorganisms to achieve obvious inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
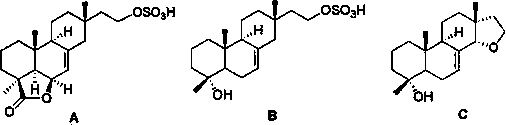
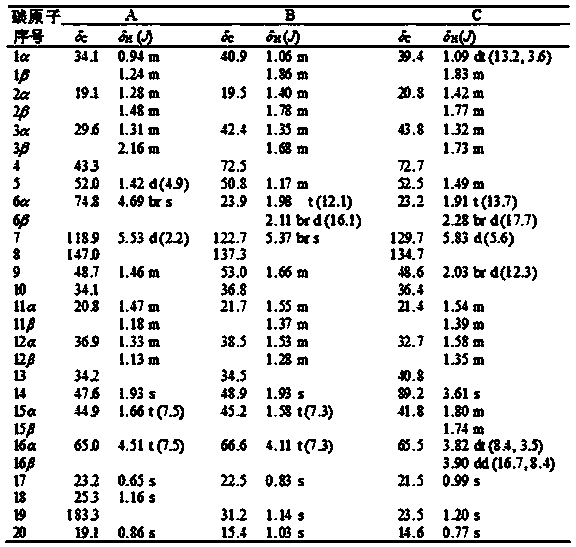
[0019] The separation and preparation of compounds A, B and C:
[0020] 1. Slant culture of Xylaria sp. YM311647 strain: peel potatoes, cut into small pieces, add water to boil, filter with gauze to obtain potato filtrate, then add glucose and agar, use distilled water to make up volume, sterilize to obtain slant medium; Make the slant medium into a test tube slant, pick the YM311647 bacterial strain and insert it into the slant medium for cultivation to obtain the slant bacterial strain;
[0021] 2. Seed culture of Xanthomonas YM311647 strain: Take a strain graft block from the above slant strains and inoculate it into PDB medium (PDB medium: 200 g of peeled potatoes, 20 g of glucose, 15 g of agar, 1000 mL of distilled water , natural pH, sterilized at 121°C for 30 minutes), cultured on a shaker at 28°C and 200 r / min for 3 days to obtain seed liquid;
[0022] 3. The liquid fermentation culture of the Xylella cerevisiae YM311647 strain: 200 mL of PDB medium was placed in a...
Embodiment 2
[0025] The antibacterial activity of the isopimarane diterpene compound of the present invention, that is, compounds A, B and C, was detected by a micro-double dilution method.
[0026] 1. A total of five strains of pathogenic indicator bacteria: including 2 strains of human pathogenic fungi: Candida albicans ( Candida albicans ) and Cladomonospora ( Hormodendrum compactum ); 3 plant pathogenic fungi: Aspergillus niger ( Aspergillus niger ), Magnaporthe oryzae ( Pyricularia oryzae ) and Fusarium avenae ( Fusarium avenaceum ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com