Novel prescription composition and preparation method of paroxetine hydrochloride enteric controlled release tablet
A technology of paroxetine hydrochloride and controlled-release tablets, applied in the field of medicine, can solve the problems of large adverse reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
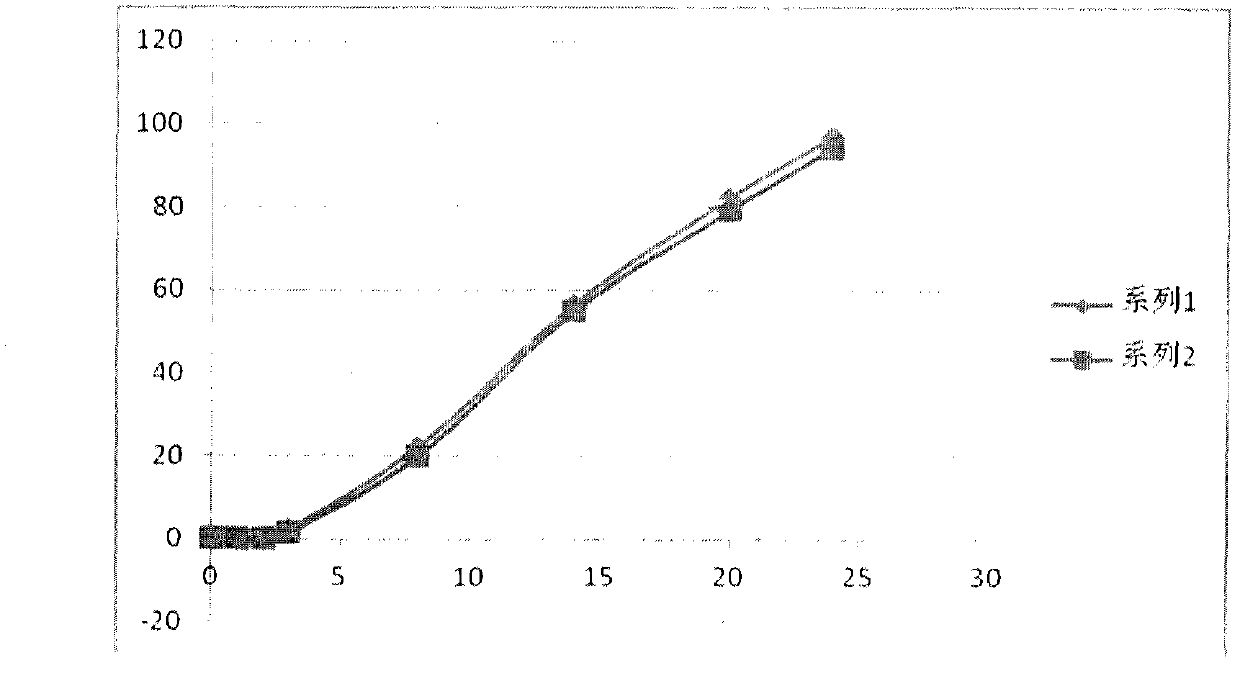
Image
Examples
Embodiment 1
[0015] 1. Prescription:
[0016] Hydrophilic matrix drug-containing layer
[0017] composition
Dosage
Function
25mg
Hypromellose K4M
22.5mg
Controlled release matrix
85mg
Thinner
2.5mg
2.5mg
[0018] Corrosion layer
[0019] composition
Dosage
Function
25mg
Thinner
30mg
Thinner
5mg
controlled release material
15mg
controlled release material
3mg
1mg
[0020] Enteric coating layer
[0021] composition
Dosage
Function
Eudragit L30D-55 (30% solids)
50mg
Thinner
Embodiment 2
[0033] 1. Prescription:
[0034] Hydrophilic matrix drug-containing layer
[0035] composition
[0036] Corrosion layer
[0037] composition
[0038] Enteric coating layer
[0039] composition
[0040] The preparation method of paroxetine hydrochloride double-layer controlled-release tablet is the same as that in Example 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com