Pharmaceutical composition for treating and/or preventing insulin dependent diabetes mellitus and application thereof
A composition and technology for diabetes, applied in the field of compositions for the treatment and/or prevention of type I diabetes, capable of solving the problems of inhibiting organ failure, unreasonable curative effect, toxic and side effects, etc., and achieving the effect of inhibiting the occurrence of type I diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
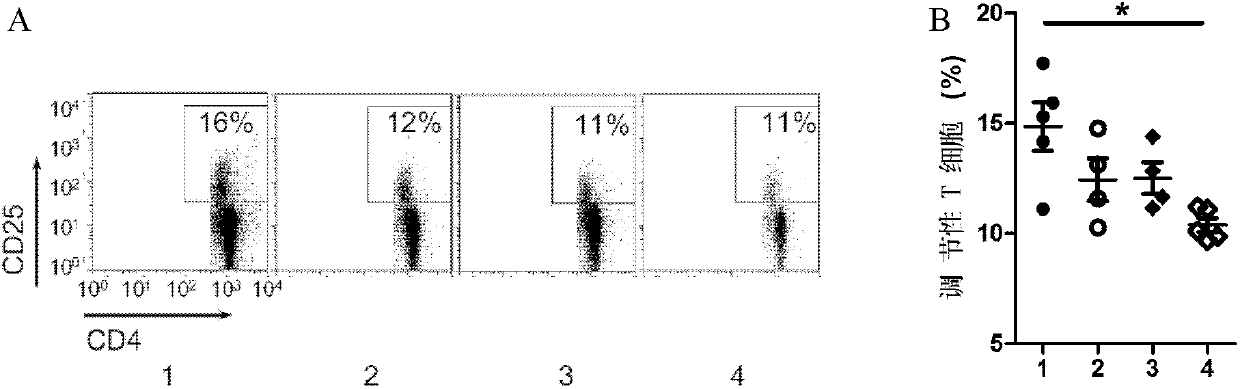
[0082] Example 1. Drug dose-effect experiment in NOD mice
[0083] 1. NOD mouse immunization
[0084] 1. Immune a pharmaceutical composition composed of human insulin (Novolin R) and dexamethasone
[0085] The NOD mice were equally divided into 4 groups, 3 in each group. On days 1, 4, and 7, the pharmaceutical composition was injected subcutaneously in the abdomen: each mouse in group 1 (group 10+10) was injected with PBS containing 10 micrograms of human insulin (Novolin R) mixed with 10 micrograms of dexamethasone 100 μl, the second group (100+100 group) each injected 100 μg insulin mixed with 100 μg dexamethasone PBS 100 μl, the third group (500+100 group) each injected 500 μg insulin mixed separately 100 micrograms of dexamethasone in 100 microliters of PBS.
[0086] 2. Immune a pharmaceutical composition composed of human insulin epitope polypeptide B9-23 and dexamethasone
[0087] The NOD mice were equally divided into 4 groups, 3 in each group. On days 1, 4, and 7, the pharma...
Embodiment 2
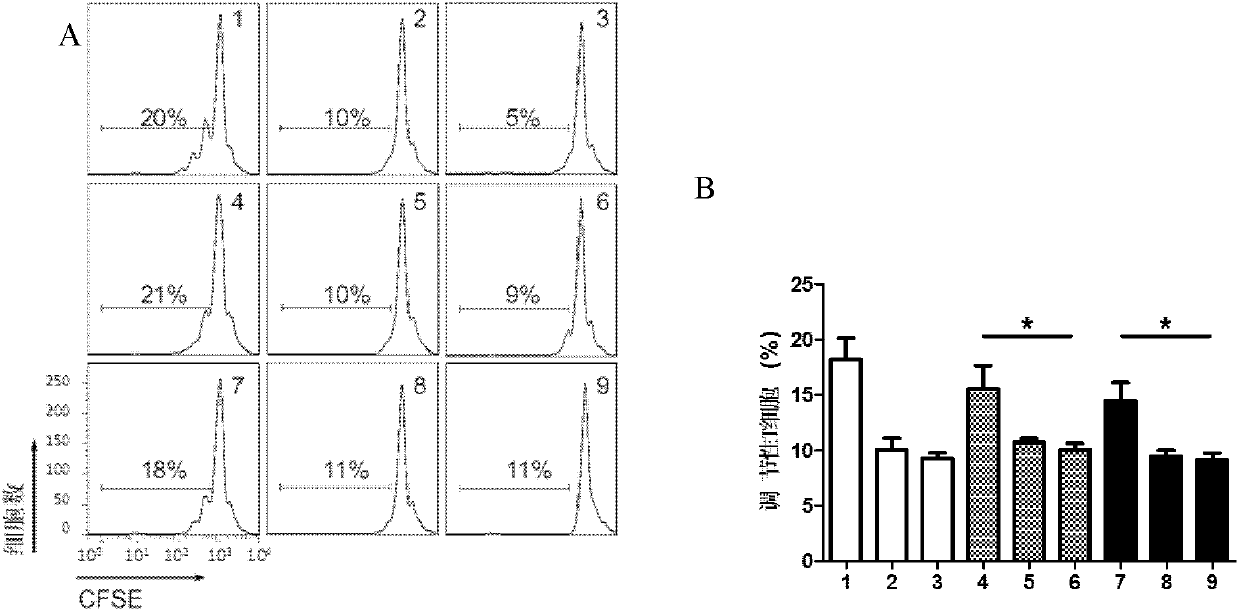
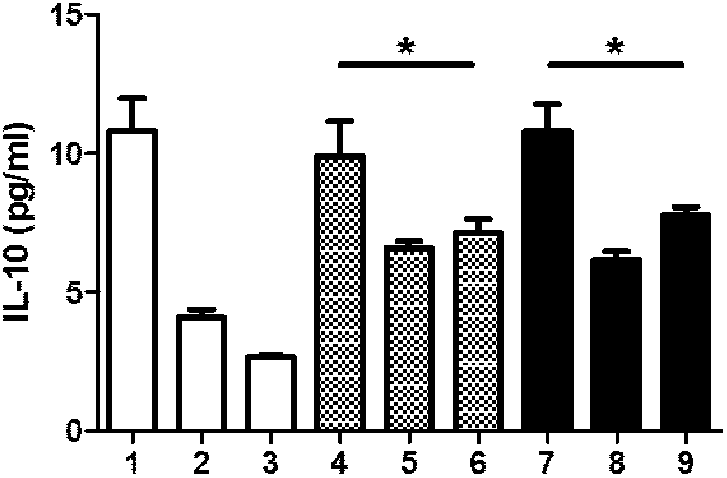
[0102] Example 2: Therapeutic effect test of immediate type I diabetes in NOD mice
[0103] 1. Pathogenesis and immunity of NOD mice
[0104] After the drug dose-effect relationship was determined in Example 1, NOD mice were used to induce type I diabetes, and after it was judged to be hyperglycemia (>12mmol), intraperitoneal injection of the drug composition (comprised of human insulin (Novoline R) and dexamethasone) was performed. Composition of the pharmaceutical composition). The specific operations are as follows:
[0105] 18 NOD mice were intraperitoneally injected with streptozotocin (STZ) (Sigma Aldrige, S0130) 40 mg / kg for 5 consecutive days to induce type I diabetes. Determined as hyperglycemia (>12m mol), (the first injection of STZ is recorded as the first day, about 10 days later, the immediate type I diabetes model of NOD mice is obtained) and then divided into 3 groups, each with 6 mice, the first No treatment was given to the group (group 10+10), the second group (...
Embodiment 3
[0120] Example 3 The treatment effect test of long-term type I diabetes in NOD mice
[0121] After Example 2 proved that the pharmaceutical composition (a pharmaceutical composition composed of human insulin (Novolin R) and dexamethasone) has a therapeutic effect on immediate type I diabetes, the treatment evaluation of the long-term type I diabetes was performed ,details as follows:
[0122] 1. Pathogenesis and immunity of NOD mice
[0123] 16 NOD mice were injected with STZ 40mg / kg intraperitoneally for 5 consecutive days to induce type I diabetes. Two months after the onset of the disease, they were injected. Divide into 2 groups, 8 animals in each group, the first group (the onset group) will not be treated, and the second group (treatment group) will be injected with 10 micrograms of human insulin mixed with 10 micrograms of dexamethasone in 100 microliters of PBS as treatment group. A subcutaneous injection in the abdomen was given on the 1, 4, and 7 days each, which was a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com