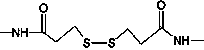Quality control object for substituting patients' positive blood
A quality control product and positive technology, applied in the field of immunology, can solve problems such as difficult standardization, difficult inactivation, and unstable quality, and achieve the effects of reducing detection errors, simple production and preparation, and avoiding infectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Use SPDP cross-linking reagent (purchased from Thermo Scientific, product number 21857) to prepare mouse anti-HSV-II McAb and healthy non-infected HSV-II human IgG heterologous cross-linked product as HSV-II in patient's positive blood Antibody surrogate, the process is as follows:
[0049] (1) Dissolve 5 mg mouse anti-HSV-II McAb in cross-linking buffer (0.1M potassium phosphate, 0.1M NaCl, pH7.5), stir and add 50 μl SPDP cross-linking agent (3.2 mg / ml, dissolved in In absolute ethanol), after reacting at room temperature for 2 hours, put it into a dialysis bag, dialyze with reducing buffer (0.1M sodium acetate, 0.1M NaCl, pH4.5), and change the solution four times;
[0050] (2) Dissolve 5mg of healthy human IgG not infected with HSV-II in the cross-linking buffer, stir and add 50μl SPDP cross-linking agent, react at room temperature for 2 hours, put it into a dialysis bag, and dialyze with the cross-linking buffer , change the liquid four times;
[0051] (3) Add 30 ...
Embodiment 2
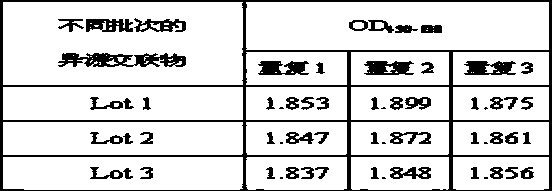
[0057] Example 2 Experiments on substitution of mouse anti-HSV-II McAb and healthy non-HSV-II-infected human IgG heterologous cross-linked product, quality control stability and cryopreservation stability
[0058] Experiments were carried out using the mouse anti-HSV-II McAb prepared in Example 1 and the heterologous cross-linked product of healthy human IgG not infected with HSV-II as a substitute for the HSV-II antibody in the patient's positive blood.
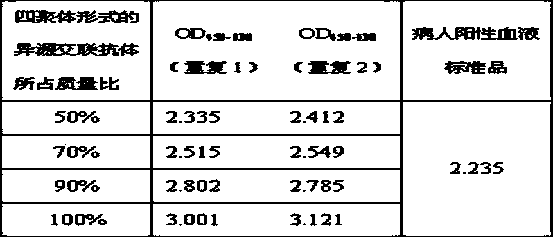
[0059] (1) Preparation of quality control products. The heterologous cross-linked products with tetrameric heterologous cross-linked antibodies accounted for 50%, 70%, 90% and 100% of the mass ratio in the prepared heterologous cross-linked products were sequentially prepared as patient Surrogates for HSV-II antibodies in positive blood. When the heterologous cross-linked antibody in tetrameric form accounts for 50%, 70% or 90% of the mass ratio of the prepared heterologous cross-linked product, the remaining 50%, 30% or 10...
Embodiment 3
[0080] Example 3 Alternative experiment of mouse anti-HSV-1 McAb and healthy non-infected HSV-1 human IgG heterologous crosslinker
[0081] Using the SPDP cross-linker to prepare mouse anti-HSV-I McAb and healthy non-HSV-I-infected human IgG heterologous cross-linked product as a surrogate for HSV-I antibody in patient-positive blood, except for mouse anti-HSV -I McAb replaces mouse anti-HSV-II McAb, and the specific process is the same as in Example 1.
[0082] Experiments were carried out using the mouse anti-HSV-1 McAb prepared in Example 3 and the heterologous cross-linked product of healthy human IgG not infected with HSV-1 as a substitute for the HSV-1 antibody in the patient's positive blood. The heterologous cross-linked products in which the mass ratio of tetrameric heterologous cross-linked antibody in the prepared heterologous cross-linked product is 50%, 70%, 90% and 100% are sequentially prepared. When the heterologous cross-linked antibody in tetrameric form acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com