Metoprolol tartrate sustained-release tablet and preparation method thereof
A technology for metoprolol tartrate and sustained-release tablets, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of complicated preparation process, high production cost and difficult release curve. control and other problems, to achieve the effect of simple preparation method, avoiding toxic and side effects, and significant slow-release characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
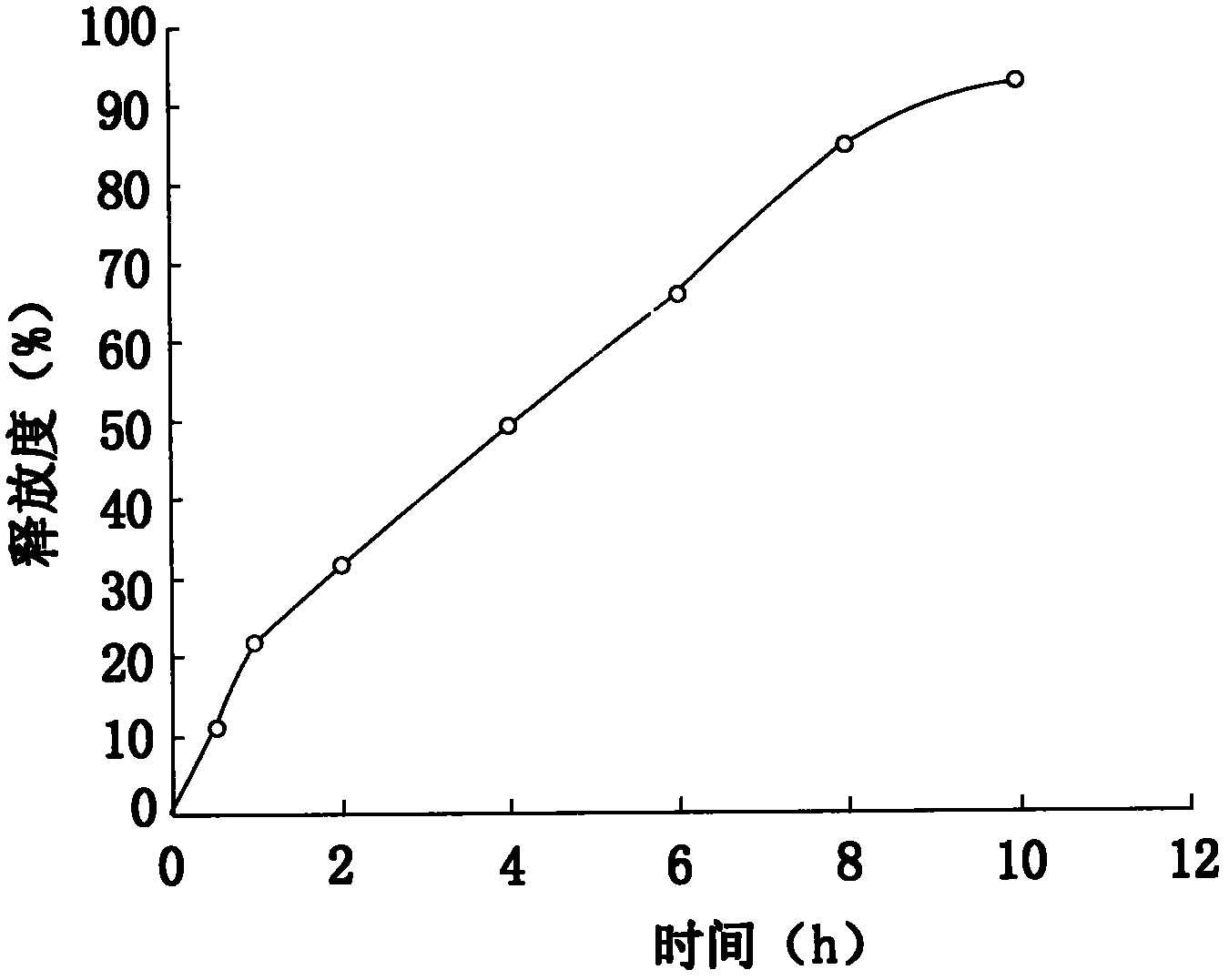
Embodiment 1
[0039] Prepare Metoprolol Tartrate Extended Release Tablets as follows:
[0040] (1) Mix metoprolol tartrate: 30%, sustained-release matrix material: 25% hypromellose, and mix for 20min to 30min to obtain a mixed powder, wherein the model of hypromellose used is K4M ;
[0041] (2) add binding agent again in the mixed powder: with the 1.2% hydroxypropyl methylcellulose 20% soft material of the preparation of 85% ethanol solution, cross 18 mesh sieves and granulate, get wet granule;
[0042] (3) Dry the wet granules in a hot air circulation oven at 50°C to 60°C until the moisture content is ≤3% to obtain dry granules;
[0043] (4) Pass the dry granules through a 16-mesh sieve for granulation; put the dry granules and lubricants: 0.8% of magnesium stearate, 1.0% of micropowder silica gel, and mix them in a mixer, and the mixing time is about 20min to 30min;
[0044] (5) The dry granules mixed with the lubricant in step (4) are placed in a tablet press, and the pressure is adjus...
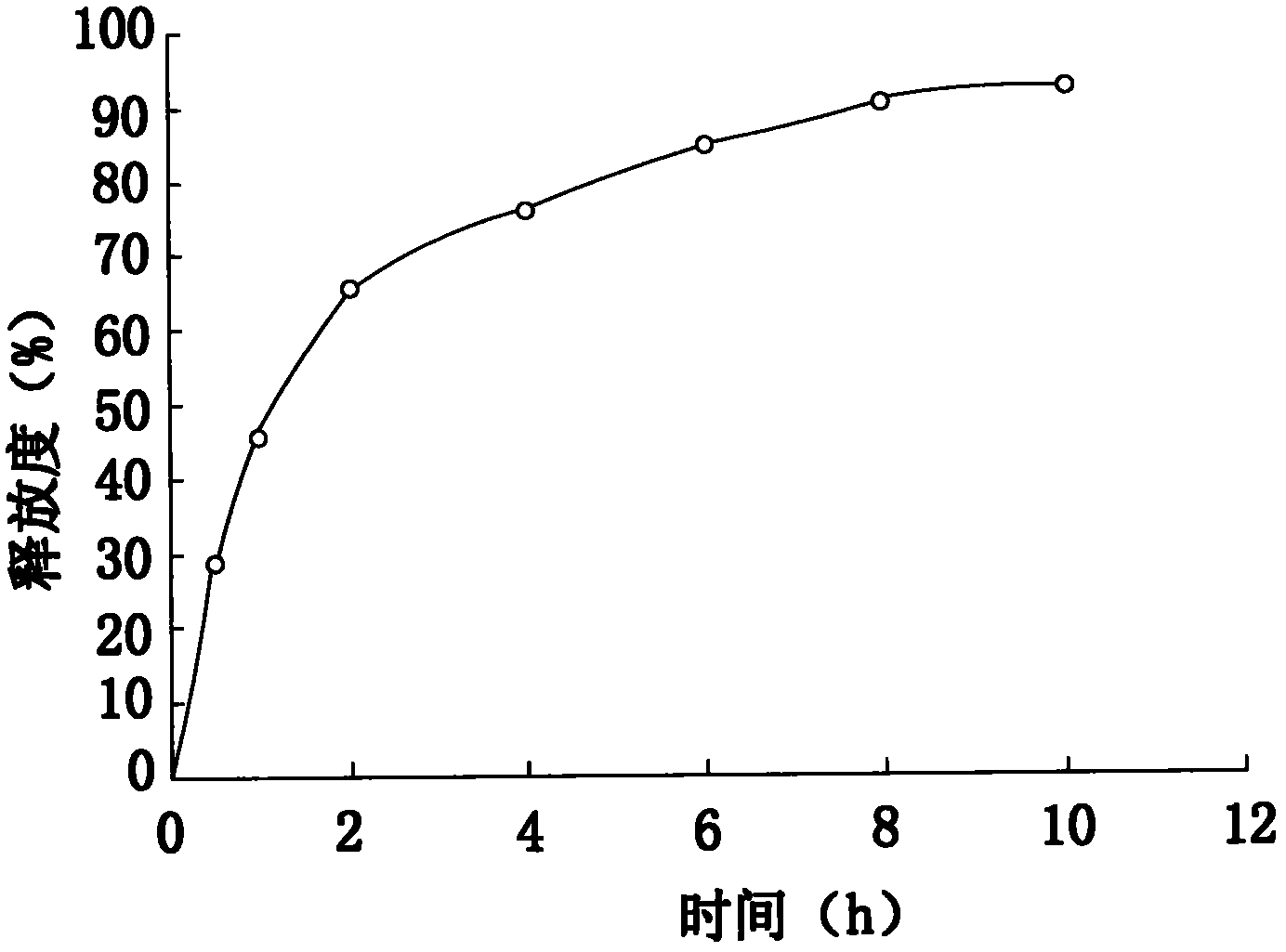
Embodiment 2
[0057] The addition amount of each raw material is: 35.0% of metoprolol tartrate, sustained-release matrix material: 20.0% of hypromellose, lubricant: 0.25% of magnesium stearate, 2.0% of micropowder silica gel, binder: 25.0%, film coating premix 2.75%, purified water 15.0%, the binder is 1.2% hydroxypropyl methylcellulose prepared with 95% ethanol solution, wherein the hydroxypropylmethylcellulose used The model of cellulose is K4M.
[0058] Its preparation method and release measurement method are the same as in Example 1.
[0059] The release measurement results are as follows:
[0060]
[0061] Finally, the release curve of this test was drawn by calculating the average release rate of the six samples in each period.
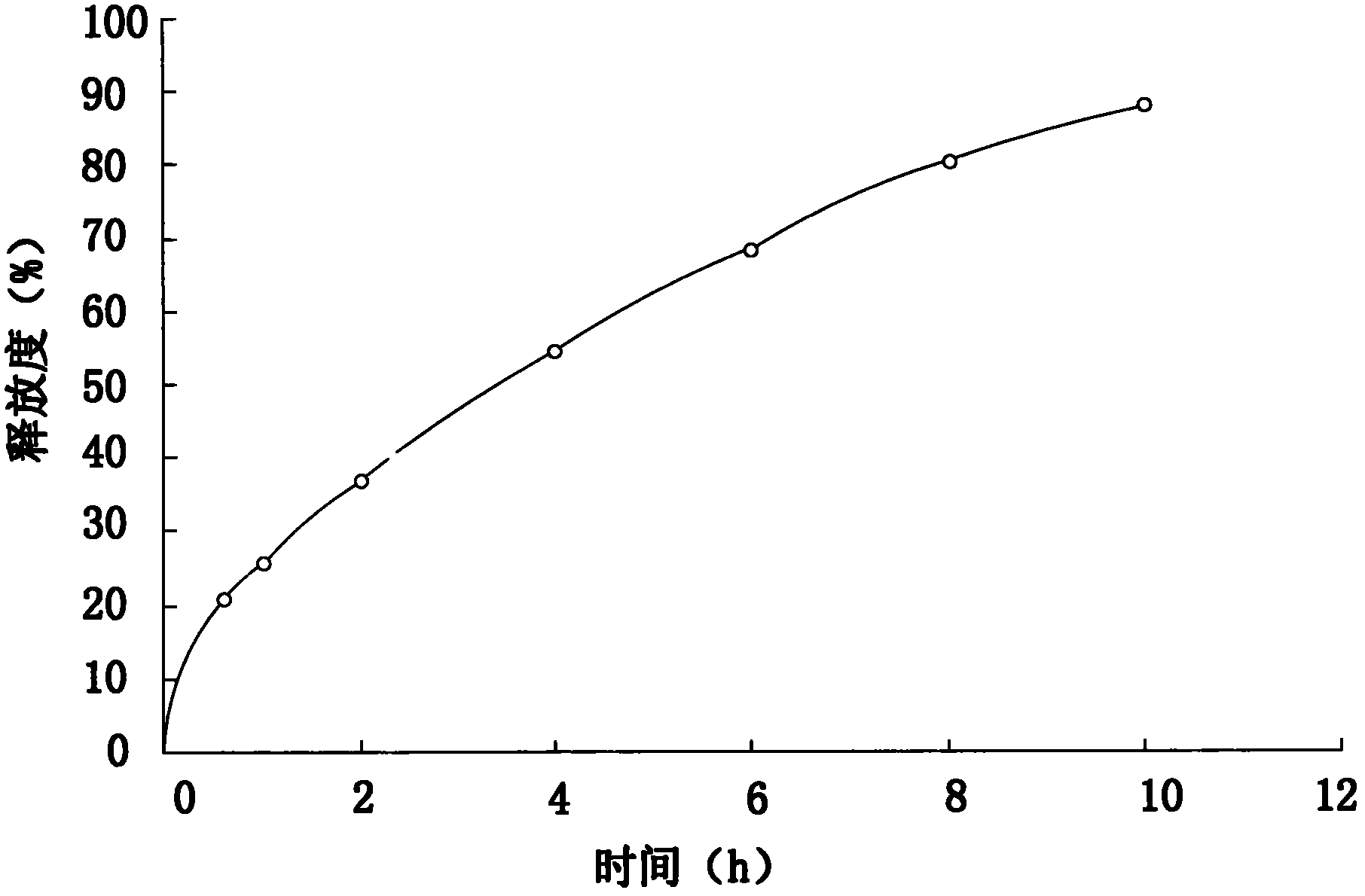
Embodiment 3
[0063] The addition amount of each raw material is: 40.0% of metoprolol tartrate, sustained-release matrix material: 25.0% of hydroxypropyl cellulose, lubricant: 2.5% of magnesium stearate, 0.1% of micropowder silica gel, binder: 15.0% %, film coating premix 1.4%, purified water 16.0%, and the binder is 1.2% hydroxypropyl methylcellulose prepared with 70% ethanol solution.
[0064] Its preparation method and release measurement method are the same as in Example 1.
[0065] The release measurement results are as follows:
[0066]
[0067] Finally, the release curve of this test was drawn by calculating the average release rate of the six samples in each period.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com