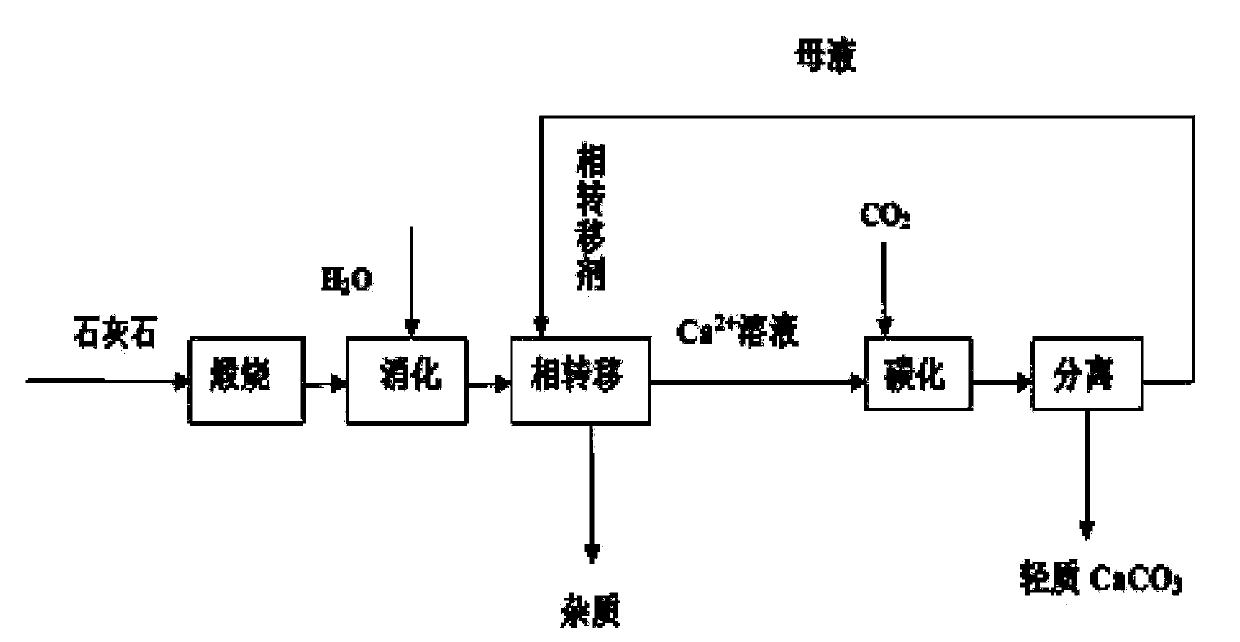
A preparation method for light calcium carbonate by using a phase transfer-carbonization method
A light calcium carbonate, phase transfer technology, applied in calcium carbonate/strontium/barium, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problem of low production efficiency, wide particle size distribution, and long process time. and other problems to achieve the effect of increasing concentration, improving production efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Add 4.58g of calcined quicklime to 100mL of water to obtain a crude quicklime slurry with a calcium ion concentration of 0.6mol / L. Stir at 300r / min for 5min at 25℃, and then according to the amount of phase transfer agent and calcium ion substance Add the phase transfer agent sodium gluconate at a ratio of 1.5:1, and continue to stir at constant temperature for 40 minutes. After the reaction is over, filter at room temperature to obtain a soluble calcium ion solution.
[0025] 2. Heat the obtained calcium ion solution to 50℃, add 0.1g isopropanol as a dispersant, and pass in CO at a rate of 40mL / min 2 Gas, stirring rate of 300r / min, constant temperature stirring reaction for 2h, the pH value of the system drops to 7.6; after the reaction, aging at 25℃ for 1h, filtering and separating the filtrate and filter cake, washing the filter cake 2-3 times with distilled water, Then dry at 60°C for 10 hours to obtain light calcium carbonate. Add the phase transfer agent to the f...
Embodiment 2
[0028] 1. Add 6.12g quicklime to 100mL water to obtain a crude quicklime slurry with a calcium ion concentration of 0.8mol / L, stir for 5min at room temperature, and then add phase transfer agent and calcium ion substance in a ratio of 1:1 The transfer agent ammonium citrate, continue to stir at constant temperature for 20 minutes. After the reaction is over, filter at room temperature to obtain a soluble calcium ion solution.
[0029] 2. Heat the resulting calcium ion solution to 30°C, add 0.15g of sodium hexametaphosphate, and pass in CO at a rate of 40mL / min 2 Gas, stirring rate is 300r / min, constant temperature stirring reaction for 2h, the pH value of the system drops to 7.5. After the completion of the reaction, it was aged at 25°C for 1 hour, filtered to separate the filtrate and filter cake, washed the filter cake 2-3 times with distilled water, and then dried at 60°C for 10 hours to obtain light calcium carbonate. Add the phase transfer agent to the filtrate and recycle ...
Embodiment 3
[0032] 1. Add 4.58g of quicklime to 100mL of water to obtain a crude quicklime slurry with a calcium ion concentration of 0.6mol / L. Stir at room temperature for 5 minutes, and then add phase transfer agent to calcium ion in a ratio of 2:1. Transfer agent lactic acid, continue to stir at constant temperature for 30 minutes. After the reaction is over, filter at room temperature to obtain a soluble calcium ion solution.
[0033] 2. Heat the resulting calcium ion solution to 30°C, add 0.1g of sodium hexametaphosphate, and pass in CO at a rate of 40mL / min 2 Gas, stirring rate of 300r / min, constant temperature stirring reaction for 2h, the pH value of the system drops to 7.6. After the completion of the reaction, it was aged at 25°C for 1 hour, filtered to separate the filtrate and filter cake, washed the filter cake 2-3 times with distilled water, and then dried at 60°C for 10 hours to obtain light calcium carbonate. Add the phase transfer agent to the filtrate and recycle it.
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com