Montelukast sodium membrane-shape preparation
A technology of montelukast sodium and film preparations, which is applied in the field of drug film preparations containing montelukast sodium, can solve the problems of mutual influence, different physical and chemical properties, influence the solubility of substance B, etc. the effect of interest
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1) Take 50g of montelukast sodium and 700ml of distilled water, stir and disperse evenly, then add PEG40004g, 20g of citric acid, 20g of stevioside and 0.25g of erythrosin in turn while stirring, and finally add 106g of HPMC, stir evenly, 80-mesh sieve to remove insoluble matter and vacuum defoaming. Obtain slurry A.
[0067] 2) Take 50g of montelukast sodium and 700ml of distilled water, stir and disperse evenly, then add PEG40004g, calcium carbonate 20g, stevioside 20g and erythrosin 0.25g in sequence while stirring, and finally add HPMC106g, stir evenly, 80-mesh sieve to remove insoluble matter and vacuum defoaming. Obtain slurry B.
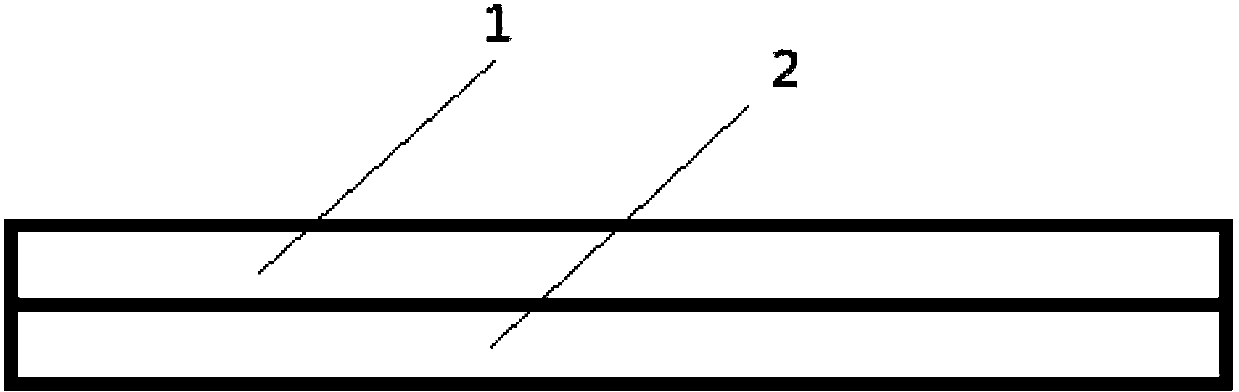
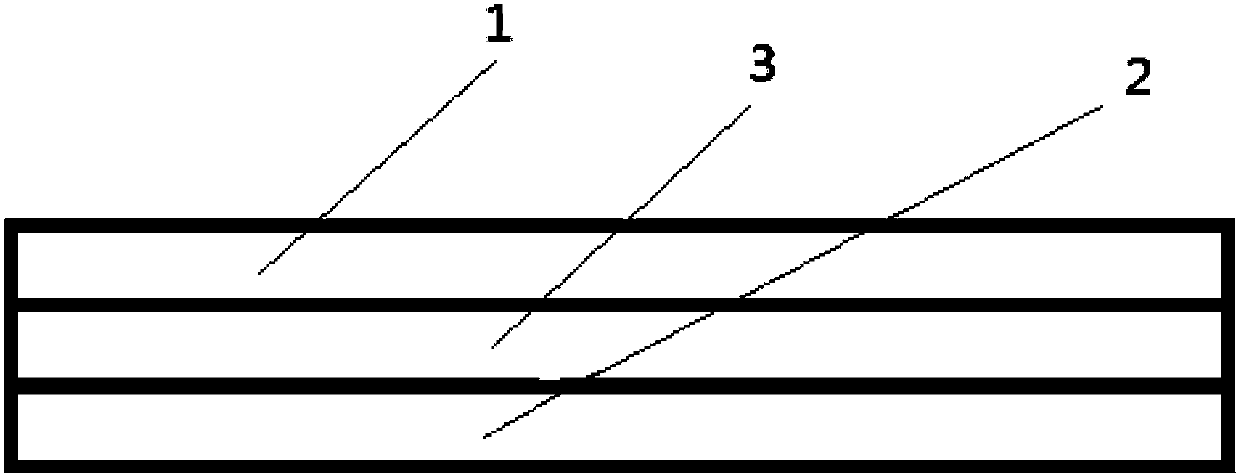
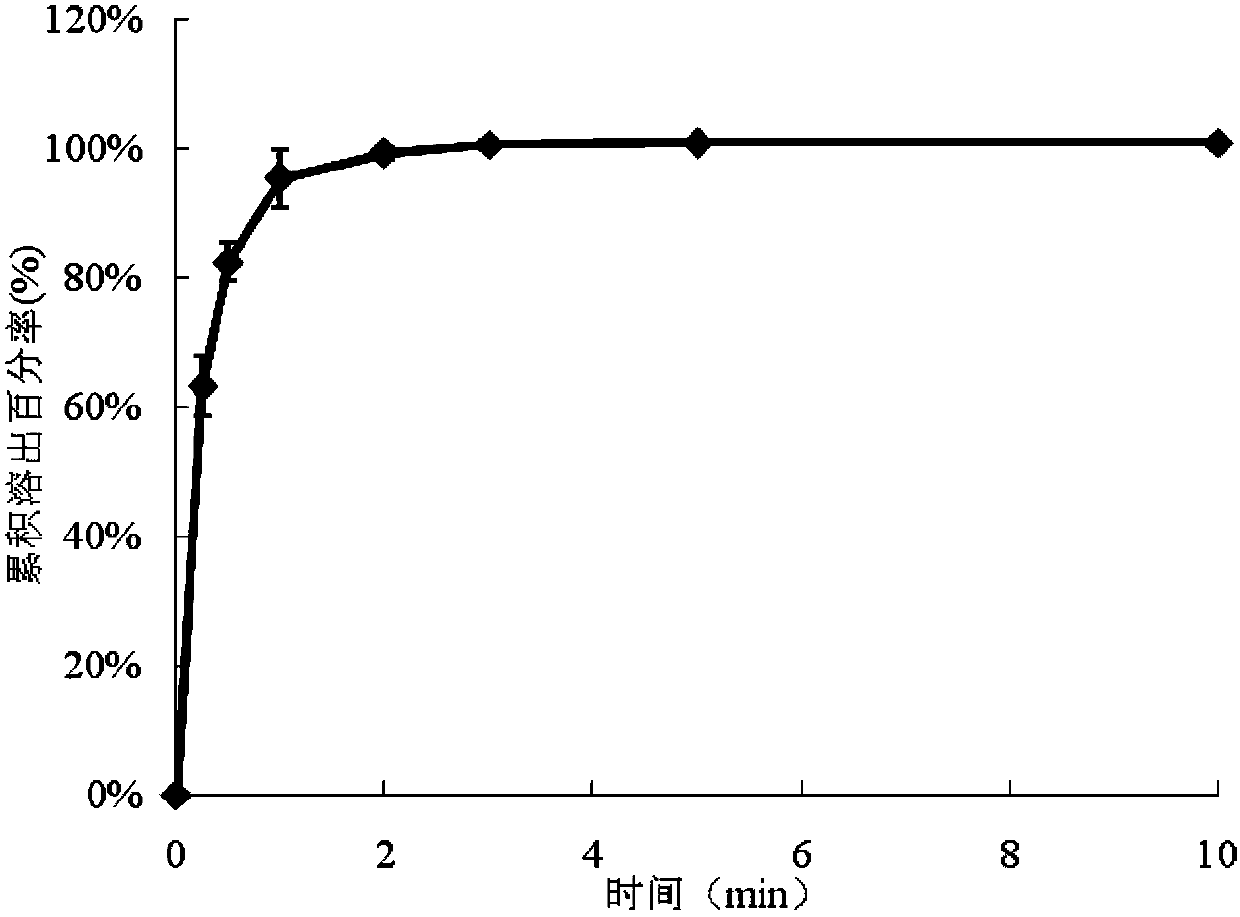
[0068] Add the slurry A and the slurry B into the dosing tank, which is composed of two parallel small tanks with a width of 1.2 cm, and the width of the partition between the small tanks is 0.1 cm. Start the coating dryer, coat with a coating blade, spread the film on a stainless steel belt, and dry at 80°C for 10 minutes. Due to th...
Embodiment 2
[0074] 1) Take 26g of montelukast sodium and add 800ml of distilled water, stir to disperse evenly, then add 10g of PEG400, 4g of tartaric acid, 10g of sodium saccharin and 0.2g of erythrosin in sequence while stirring, and finally add 100g of HPC and 50g of PVP, stir evenly, 80-mesh sieve to remove insoluble matter and vacuum defoaming. Obtain slurry A.
[0075] 2) Take 26g of montelukast sodium and add 800ml of distilled water, stir and disperse evenly, then add 10g of PEG400, 4g of sodium carbonate, 10g of sodium saccharin and 0.2g of allura red pigment in turn while stirring, and finally add 100g of HPC and 50g of PVP, stir at high speed, 80-mesh sieve to remove insoluble matter and vacuum defoaming. Obtain slurry B.
[0076] Add the slurry A and the slurry B into the dosing tank, which is composed of two parallel small tanks with a width of 1.2 cm, and the width of the partition between the small tanks is 0.1 cm. Start the coating dryer, coat with a coating blade, spre...
Embodiment 3
[0082] 1) Take 10g of montelukast sodium and 500ml of distilled water, stir and disperse evenly, then add 10g of acesulfame potassium, 20g of cyclamate, 10g of titanium dioxide, 2g of vitamin E and 15g of fumaric acid while stirring, and finally add 100g of PEO and 33g of maltodextrin , stir evenly, pass through an 80-mesh sieve, remove insoluble matter, and vacuum defoam. Obtain slurry A.
[0083] 2) Take 60g of montelukast sodium and 600ml of distilled water, stir, and disperse evenly, then add 15g of aspartame, 10g of titanium dioxide, and 15g of potassium bicarbonate while stirring, and finally add 100g of PEO, stir evenly, pass through an 80-mesh sieve, and remove Insoluble matter, vacuum defoaming. Obtain slurry B.
[0084] Add the slurry A and the slurry B into the dosing tank, which is composed of two parallel small tanks with a width of 1.2 cm, and the width of the partition between the small tanks is 0.1 cm. Start the coating dryer, coat with a coating blade, spre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com